
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
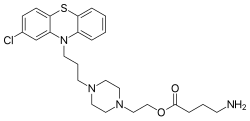
| Formula | C25H33ClN4O2S |
| Molar mass | 489.08 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
BL-1020 (perphenazine 4-aminobutanoate) is an investigational orally-active antipsychotic for the possible treatment of schizophrenia. The chemical is an ester of GABA and perphenazine; pharmacologically it acts as a D2 antagonist and GABA agonist. It has shown pro-cognitive effects in the trials.[1] In March 2013, it went into the II/III trial phase.[2] It has been introduced by BioLineRx, a biopharmaceutical development company.
Referencesedit
- ^ Geffen Y, Keefe R, Rabinowitz J, Anand R, Davidson M (September 2012). "Bl-1020, a new γ-aminobutyric acid-enhanced antipsychotic: results of 6-week, randomized, double-blind, controlled, efficacy and safety study". The Journal of Clinical Psychiatry. 73 (9): e1168-74. doi:10.4088/jcp.12m07642. PMID 23059159.
- ^ Biolinerx (Jan 7, 2013). "BioLineRx to Announce Interim Results of Phase II/III Trial for Schizophrenia Drug during week of March 18, 2013". Biolinerx. Archived from the original on 2013-03-03.
| Ionotropic |
| ||||
|---|---|---|---|---|---|
| Metabotropic |
| ||||
| 5-HT1 |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
