
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
The Corey–Winter olefin synthesis (also known as Corey–Winter–Eastwood olefination) is a series of chemical reactions for converting 1,2-diols into olefins.[1][2][3][4] It is named for the American chemist and Nobelist Elias James Corey and the American-Estonian chemist Roland Arthur Edwin Winter.[5]

Often, thiocarbonyldiimidazole is used instead of thiophosgene as shown above, since thiophosgene has a similar toxicity profile as phosgene, whereas thiocarbonyldiimidazole is a much safer alternative.
Mechanism
The reaction mechanism involves the formation of a cyclic thiocarbonate from the diol and thiophosgene. The second step involves treatment with trimethyl phosphite, which attacks the sulfur atom, producing S=P(OMe)3 (driven by the formation of a strong P=S double bond) and leaving a carbene.[6] This carbene collapses with loss of carbon dioxide to give the olefin.
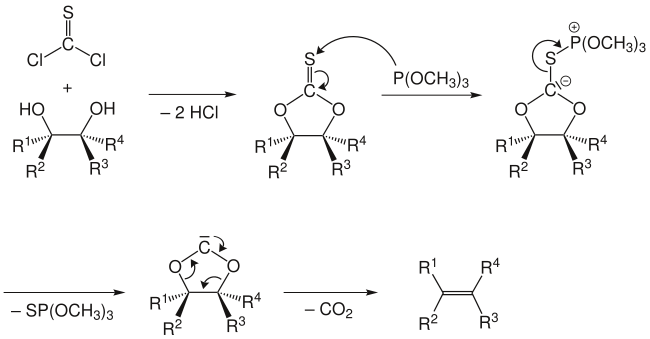
An alternative mechanism does not involve a free carbene intermediate, but rather involves attack of the carbanion by a second molecule of trimethylphosphite with concomitant cleavage of the sulfur-carbon bond. The phosphorus stabilized carbanion then undergoes an elimination to give the alkene, along with an acyl phosphite, which then decarboxylates.

The Corey-Winter olefination is a stereospecific reaction:[1] a trans-diol gives a trans-alkene, while a cis-diol gives a cis-alkene as the product. For instance, cis- and trans-1,2-cyclodecanediol gives the respective cis- and trans-cyclodecene.
References
- ^ a b Corey, E. J.; Winter, R. A. E. (1963). "A New, Stereospecific Olefin Synthesis from 1,2-Diols". J. Am. Chem. Soc. 85 (17): 2677–2678. doi:10.1021/ja00900a043.
- ^ Corey, E. J.; Carey, F. A.; Winter, R. A. E. (1965). "Stereospecific Syntheses of Olefins from 1,2-Thionocarbonates and 1,2-Trithiocarbonates. trans-Cycloheptene". J. Am. Chem. Soc. 87 (4): 934–935. doi:10.1021/ja01082a057.
- ^ Corey, E. J.; Hopkins, J. (1982). "A mild procedure for the conversion of 1,2-diols to olefins". Tetrahedron Lett. 23 (19): 1979–1982. doi:10.1016/S0040-4039(00)87238-X.
- ^ Crank, G.; Eastwood, F. W. (1964). "Derivatives of orthoacids. II. The preparation of olefins from 1,2-diols". Australian Journal of Chemistry. 17 (12): 1392–1398. doi:10.1071/CH9641392.
- ^ Block, E. Org. React. 1984, 30, 457. doi:10.1002/0471264180.or030.02
- ^ Horton, D.; Tindall, Jr., C. G. J. Org. Chem. 1970, 35(10), 3558-3559. (doi:10.1021/jo00835a082)
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk
