
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
The Curtin–Hammett principle is a principle in chemical kinetics proposed by David Yarrow Curtin and Louis Plack Hammett. It states that, for a reaction that has a pair of reactive intermediates or reactants that interconvert rapidly (as is usually the case for conformational isomers), each going irreversibly to a different product, the product ratio will depend both on the difference in energy between the two conformers and the energy barriers from each of the rapidly equilibrating isomers to their respective products. Stated another way, the product distribution reflects the difference in energy between the two rate-limiting transition states. As a result, the product distribution will not necessarily reflect the equilibrium distribution of the two intermediates.[1][2] The Curtin–Hammett principle has been invoked to explain selectivity in a variety of stereo- and regioselective reactions. The relationship between the (apparent) rate constants and equilibrium constant is known as the Winstein-Holness equation.
Definition
The Curtin–Hammett principle applies to systems in which different products are formed from two substrates in equilibrium with one another. The rapidly interconverting reactants can have any relationship between themselves (stereoisomers, constitutional isomers, conformational isomers, etc.). Product formation must be irreversible, and the different products must be unable to interconvert.[3]
For example, given species A and B that equilibrate rapidly while A turns irreversibly into C, and B turns irreversibly into D:
K is the equilibrium constant between A and B, and k1 and k2 are the rate constants for the formation of C and D, respectively. When the rate of interconversion between A and B is much faster than either k1 or k2, then the Curtin–Hammett principle tells us that the C:D product ratio is not equal to the equilibrium A:B reactant ratio, but is instead determined by the relative energies of the transition states (i.e., difference in the absolute energies of the transition states). If reactants A and B were at identical energies, the product ratio would depend only on the activation barriers of the reactions leading to each respective product. However, in a real-world scenario, the two reactants are likely at somewhat different energy levels, although the barrier to their interconversion must be low for the Curtin–Hammett scenario to apply. In this case, the product distribution depends both on the equilibrium ratio of A to B and on the relative activation barriers going to the corresponding products C and D. Both factors are taken into account by the difference in the energies of the transition states (ΔΔG‡ in the figure below).
The reaction coordinate free energy profile of a typical reaction under Curtin-Hammett control is represented by the following figure:
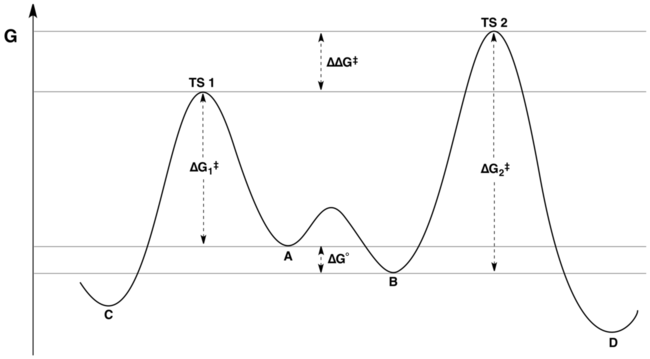
The ratio of products only depends on the value labeled ΔΔG‡ in the figure: C will be the major product, because the energy of TS1 is lower than the energy of TS2. A common but false assertion is that the product distribution does not in any way reflect the relative free energies of substrates A and B; in fact, it reflects the relative free energies of the substrates and the relative activation energies.[3][4] This misunderstanding may stem from failing to appreciate the distinction between "the difference of energies of activation" and "the difference in transition state energies". Although these quantities may at first appear synonymous, the latter takes into account the equilibrium constant for interconversion of A and B, while the former does not.
Mathematically, the product ratio can be expressed as a function of K, k1, and k2 or in terms of the corresponding energies ΔG°, ΔG1‡, and ΔG2‡. By combining terms, the product ratio can be rewritten in terms of the quantity ΔΔG‡ alone, where ΔΔG‡ = (ΔG2‡ – ΔG1‡) + ΔG°. Inspection of the energy diagram (shown above) makes it apparent that ΔΔG‡ is precisely the difference in transition state energies.
Derivation
A generic reaction under Curtin–Hammett can be described by the following parameters:
In order for rapid equilibration to be a good assumption, the rate of conversion from the less stable of A or B to the product C or D must be at least 10 times slower than the rate of equilibration between A and B.[5]
The rate of formation for compound C from A is given as
- ,
and that of D from B as
- ,
with the second approximate equality following from the assumption of rapid equilibration. Under this assumption, the ratio of the products is then
- .
In other words, because equilibration is fast compared to product formation, throughout the reaction. As a result, also remains roughly constant throughout the reaction. In turn, integration with respect to time implies that likewise takes on an approximately constant value through the course of the reaction, namely .
In terms of the ground state and transition state energies, the product ratio can therefore be written as:
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk







