
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| Vaccine description | |
|---|---|
| Target | SARS-CoV-2 |
| Vaccine type | mRNA, viral, inactivated, protein |
| Clinical data | |
| Routes of administration | Intramuscular |
| ATC code | |
| Identifiers | |
| ChemSpider |
|
| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|
A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).
Prior to the COVID‑19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). This knowledge accelerated the development of various vaccine platforms in early 2020.[1] The initial focus of SARS-CoV-2 vaccines was on preventing symptomatic, often severe, illness.[2] In 2020, the first COVID‑19 vaccines were developed and made available to the public through emergency authorizations[3] and conditional approvals.[4][5] Initially, most COVID‑19 vaccines were two-dose vaccines, with the sole exception being the single-dose Janssen COVID‑19 vaccine.[3] However, immunity from the vaccines has been found to wane over time, requiring people to get booster doses of the vaccine to maintain protection against COVID‑19.[3]
The COVID‑19 vaccines are widely credited for their role in reducing the spread of COVID‑19 and reducing the severity and death caused by COVID‑19.[3][6] According to a June 2022 study, COVID‑19 vaccines prevented an additional 14.4 to 19.8 million deaths in 185 countries and territories from 8 December 2020 to 8 December 2021.[7][8] Many countries implemented phased distribution plans that prioritized those at highest risk of complications, such as the elderly, and those at high risk of exposure and transmission, such as healthcare workers.[9][10]
Common side effects of COVID‑19 vaccines include soreness, redness, rash, inflammation at the injection site, fatigue, headache, myalgia (muscle pain), and arthralgia (joint pain), which resolve without medical treatment within a few days.[11][12] COVID‑19 vaccination is safe for people who are pregnant or are breastfeeding.[13]
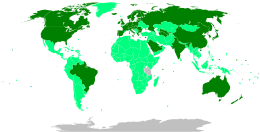
As of 1 February 2024[update], 13.57 billion doses of COVID‑19 vaccines have been administered worldwide, based on official reports from national public health agencies.[14] By December 2020, more than 10 billion vaccine doses had been preordered by countries,[15] with about half of the doses purchased by high-income countries comprising 14% of the world's population.[16]
Despite the extremely rapid development of effective mRNA and viral vector vaccines, worldwide vaccine equity has not been achieved. The development and use of whole inactivated virus (WIV) and protein-based vaccines have also been recommended, especially for use in developing countries.[17][18]
The 2023 Nobel Prize in Physiology or Medicine was awarded to Katalin Karikó and Drew Weissman for the development of effective mRNA vaccines against COVID-19.[19][20][21]
Background
-
COVID‑19 vaccine doses administered by continent as of October 11, 2021. For vaccines that require multiple doses, each individual dose is counted. As the same person may receive more than one dose, the number of doses can be higher than the number of people in the population.
-
Map showing share of population fully vaccinated against COVID-19 relative to a country's total population[note 1]

Prior to COVID‑19, a vaccine for an infectious disease had never been produced in less than several years – and no vaccine existed for preventing a coronavirus infection in humans.[22] However, vaccines have been produced against several animal diseases caused by coronaviruses, including (as of 2003) infectious bronchitis virus in birds, canine coronavirus, and feline coronavirus.[23] Previous projects to develop vaccines for viruses in the family Coronaviridae that affect humans have been aimed at severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Vaccines against SARS[24] and MERS[25] have been tested in non-human animals.
According to studies published in 2005 and 2006, the identification and development of novel vaccines and medicines to treat SARS was a priority for governments and public health agencies around the world at that time.[26][27][28] There is no cure or protective vaccine proven to be safe and effective against SARS in humans.[29][30] There is also no proven vaccine against MERS.[31] When MERS became prevalent, it was believed that existing SARS research might provide a useful template for developing vaccines and therapeutics against a MERS-CoV infection.[29][32] As of March 2020, there was one (DNA-based) MERS vaccine that completed Phase I clinical trials in humans,[33] and three others in progress, all being viral-vectored vaccines: two adenoviral-vectored (ChAdOx1-MERS, BVRS-GamVac) and one MVA-vectored (MVA-MERS-S).[34]
Vaccines that use an inactive or weakened virus that has been grown in eggs typically take more than a decade to develop.[35][36] In contrast, mRNA is a molecule that can be made quickly, and research on mRNA to fight diseases was begun decades before the COVID‑19 pandemic by scientists such as Drew Weissman and Katalin Karikó, who tested on mice. Moderna began human testing of an mRNA vaccine in 2015.[35] Viral vector vaccines were also developed for the COVID‑19 pandemic after the technology was previously cleared for Ebola.[35]
As multiple COVID‑19 vaccines have been authorized or licensed for use, real-world vaccine effectiveness (RWE) is being assessed using case control and observational studies.[37][38] A study is investigating the long-lasting protection against SARS-CoV-2 provided by the mRNA vaccines.[39][40]
Vaccine technologies
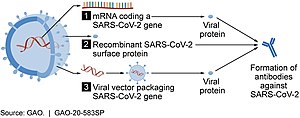
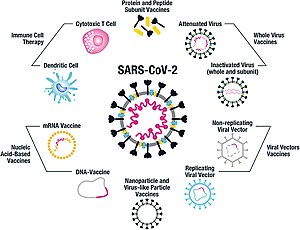
As of July 2021, at least nine different technology platforms were under research and development to create an effective vaccine against COVID‑19.[42][43] Most of the platforms of vaccine candidates in clinical trials are focused on the coronavirus spike protein (S protein) and its variants as the primary antigen of COVID‑19 infection,[42] since the S protein triggers strong B-cell and T-cell immune responses.[44][45] However, other coronavirus proteins are also being investigated for vaccine development, like the nucleocapsid, because they also induce a robust T-cell response and their genes are more conserved and recombine less frequently (compared to Spike).[45][46][47] Future generations of COVID‑19 vaccines that may target more conserved genomic regions will also act as insurance against the manifestation of catastrophic scenarios concerning the future evolutionary path of SARS-CoV-2, or any similar coronavirus epidemic/pandemic.[48]
Platforms developed in 2020 involved nucleic acid technologies (nucleoside-modified messenger RNA and DNA), non-replicating viral vectors, peptides, recombinant proteins, live attenuated viruses, and inactivated viruses.[22][42][49][50]
Many vaccine technologies being developed for COVID‑19 are not like influenza vaccines but rather use "next-generation" strategies for precise targeting of COVID‑19 infection mechanisms.[42][49][50] Several of the synthetic vaccines use a 2P mutation to lock the spike protein into its prefusion configuration, stimulating an adaptive immune response to the virus before it attaches to a human cell.[51] Vaccine platforms in development may improve flexibility for antigen manipulation and effectiveness for targeting mechanisms of COVID‑19 infection in susceptible population subgroups, such as healthcare workers, the elderly, children, pregnant women, and people with weakened immune systems.[42][49]
mRNA vaccines

Several COVID‑19 vaccines, such as the Pfizer–BioNTech and Moderna vaccines, use RNA to stimulate an immune response. When introduced into human tissue, the vaccine contains either self-replicating RNA or messenger RNA (mRNA), which both cause cells to express the SARS-CoV-2 spike protein. This teaches the body how to identify and destroy the corresponding pathogen. RNA vaccines often use nucleoside-modified messenger RNA. The delivery of mRNA is achieved by a coformulation of the molecule into lipid nanoparticles, which protect the RNA strands and help their absorption into the cells.[52][53][54][55]
RNA vaccines are the first COVID‑19 vaccines to be authorized in the United Kingdom, the United States, and the European Union.[56][57] Authorized vaccines of this type are the Pfizer–BioNTech
Severe allergic reactions are rare. In December 2020, 1,893,360 first doses of Pfizer–BioNTech COVID‑19 vaccine administration resulted in 175 cases of severe allergic reactions, of which 21 were anaphylaxis.[64] For 4,041,396 Moderna COVID‑19 vaccine dose administrations in December 2020 and January 2021, only ten cases of anaphylaxis were reported.[64] Lipid nanoparticles (LNPs) were most likely responsible for the allergic reactions.[64]
Adenovirus vector vaccines
These vaccines are examples of non-replicating viral vector vaccines using an adenovirus shell containing DNA that encodes a SARS‑CoV‑2 protein.[65][66] The viral vector-based vaccines against COVID‑19 are non-replicating, meaning that they do not make new virus particles but rather produce only the antigen that elicits a systemic immune response.[65]
Authorized vaccines of this type are the Oxford–AstraZeneca COVID‑19 vaccine,
Convidecia and Janssen are both one-shot vaccines that offer less complicated logistics and can be stored under ordinary refrigeration for several months.[73][74]
Sputnik V uses Ad26 for its first dose, which is the same as Janssen's only dose, and Ad5 for the second dose, which is the same as Convidecia's only dose.[75]
In August 2021, the developers of Sputnik V proposed, in view of the Delta case surge, that Pfizer test the Ad26 component (termed its 'Light' version)[76] as a booster shot.[77]
Inactivated virus vaccines
Inactivated vaccines consist of virus particles that are grown in culture and then killed using a method such as heat or formaldehyde to lose disease-producing capacity while still stimulating an immune response.[78]
Inactivated virus vaccines authorized in China include the Chinese CoronaVac[79][80][81] and the Sinopharm BIBP
Subunit vaccines
Subunit vaccines present one or more antigens without introducing whole pathogen particles. The antigens involved are often protein subunits, but they can be any molecule fragment of the pathogen.[88]
The authorized vaccines of this type are the peptide vaccine EpiVacCorona,
The V451 vaccine was in clinical trials that were terminated after it was found that the vaccine may potentially cause incorrect results for subsequent HIV testing.
Virus-like particle vaccines
The authorized vaccines of this type include the Novavax COVID‑19 vaccine.[17][101]
Other types
Additional types of vaccines that are in clinical trials include multiple DNA plasmid vaccines,[102]
Scientists investigated whether existing vaccines for unrelated conditions could prime the immune system and lessen the severity of COVID‑19 infections.[111] There is experimental evidence that the BCG vaccine for tuberculosis has non-specific effects on the immune system, but there is no evidence that this vaccine is effective against COVID‑19.[112]
List of authorized vaccines
| Common name | Type (technology) | Country of origin | First authorization | Notes |
|---|---|---|---|---|
| Authorized in more than 10 countries | ||||
| Oxford–AstraZeneca | Adenovirus vector | United Kingdom, Sweden | December 2020 | |
| Pfizer–BioNTech | RNA | Germany, United States |
December 2020 | Both original and Omicron variant versions |
| Janssen (Johnson & Johnson) | Adenovirus vector | United States, Netherlands |
February 2021 | |
| Moderna | RNA | United States | December 2020 | Both original and Omicron variant versions |
| Sinopharm BIBP | Inactivated | China | July 2020 | |
| Sputnik V | Adenovirus vector | Russia | August 2020 | |
| CoronaVac | Inactivated | China | August 2020 | Zdroj:https://en.wikipedia.org?pojem=COVID-19_vaccine|


