A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
National regulatory authorities have granted full or emergency use authorizations for 40 COVID-19 vaccines.
Ten vaccines have been approved for emergency or full use by at least one stringent regulatory authority recognized by the World Health Organization (WHO): Pfizer–BioNTech, Oxford–AstraZeneca, Sinopharm BIBP, Moderna, Janssen, CoronaVac, Covaxin, Novavax, Convidecia, and Sanofi–GSK.[1] Seven others are under assessment by the WHO: Sputnik V, Sinopharm WIBP, Abdala, Zifivax, Corbevax, COVIran Barekat, and SCB-2019.[2]
Of the 40 vaccines, 16 have a full or emergency authorization in only one country, 12 in ten or fewer countries, and 12 in more than ten countries.
Note that in some countries, vaccines may be authorized solely for travel purposes. They may not be approved for the general population. For example, the CoronaVac, Covishield, BBIBP-CorV and Covaxin vaccines are not part of Australia's national vaccination program; however, they are recognized for the purpose of travel to Australia.[3][4][5]
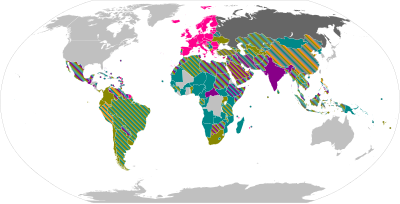
Overview maps
|
Oxford–AstraZeneca
Full authorization
Emergency authorization
Allowed for travel
Eligible COVAX recipient
Usage stopped | |||
The Oxford–AstraZeneca COVID-19 vaccine, sold under the brand names Vaxzevria[6] and Covishield,[7] is a viral vector vaccine[8] produced by the British University of Oxford, British-Swedish company AstraZeneca, and the Coalition for Epidemic Preparedness Innovations.[8][9][10] Finland, Denmark, and Norway suspended the use of the Oxford–AstraZeneca vaccine due to a small number of reports of a rare blood clot disorder.[11][12][13][14] Slovakia suspended its use after the death of a predisposed recipient.[15] South Africa suspended its use because a small trial found only minimal protection against mild to moderate disease from the locally predominant Beta variant.[16] Japan approved the vaccine for emergency use in May 2021, but did not plan to use them immediately because of rare cases of a blood clotting disorder reported overseas.[17] Later, Japan started to use the vaccine for people aged 40 or over to mitigate the surge of the Delta variant in August.[18] Finland ceased use of the vaccine as the last batch expired on 30 November 2021. Until then it was only offered for those aged 65 or more due to extremely rare coagulation disorders among younger recipients of the vaccine. After this Finland will not procure more of the vaccine.[19][20][21][22] The AstraZeneca vaccine is the most widely accepted internationally,[23] and the most popular in terms of total inoculated worldwide, over 1.3 billion.[24][25][26][27] The AstraZeneca vaccine is administered in more countries than any other vaccine.[28]
- Full (5)
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Emergency (170)
- Afghanistan[40][41]
- Albania[42]
- Algeria[43]
- Andorra[44]
- Angola[45]
- Argentina[46]
- Armenia[47]
- Azerbaijan[48]
- Bahrain[49]
- Bangladesh[50][51]
- Benin[48]
- Bhutan[52][53]
- Bolivia[48]
- Bosnia and Herzegovina[54]
- Botswana[55]
- Brunei[56]
- Burkina Faso[57]
- Cambodia[48]
- Cameroon[48]
- Cape Verde[58]
- Central African Republic[57]
- Chile[59]
- Colombia[60]
- Comoros[48]
- Congo-Brazzaville[48]
- Congo-Kinshasa[61]
- Costa Rica[62]
- Djibouti[48]
- Dominican Republic[63]
- East Timor[64]
- Ecuador[65]
- Egypt[66]
- El Salvador[67]
- Eswatini[68]
- Ethiopia[69][70][71]
- Fiji[48]
- Gambia[72]
- Georgia[73]
- Ghana[74]
- Guatemala[75]
- Guinea-Bissau[76]
- Guinea[57]
- Honduras[48]
- Indonesia[77]
- Iran[78]
- Iraq[79]
- Ivory Coast[80]
- Japan[81]
- Jordan[82]
- Kenya[83]
- Kiribati[57]
- Kosovo[84][85]
- Kuwait[86]
- Kyrgyzstan[57]
- Laos[57]
- Lebanon[87]
- Lesotho[88]
- Liberia[89]
- Libya[90][91]
- Madagascar[48]
- Malawi[92][93]
- Malaysia[94]
- Maldives[95]
- Mali[96]
- Mauritania[57]
- Mauritius[97]
- Mexico[98]
- Micronesia[57]
- Moldova[99]
- Mongolia[100]
- Montenegro[48]
- Morocco[101]
- Mozambique[48]
- Myanmar[102]
- Namibia[103]
- Nauru[104]
- Nepal[105]
- New Zealand[57]
- Nicaragua[48]
- Niger[48]
- Nigeria[106]
- North Macedonia[107]
- Oman[48]
- Pakistan[108]
- Palestine[48]
- Panama[48]
- Papua New Guinea[109][110]
- Paraguay[57]
- Peru[111]
- Philippines[112]
- Qatar[57]
- Rwanda[113]
- Samoa[104]
- São Tomé and Príncipe[48]
- Saudi Arabia[114]
- Senegal[57]
- Serbia[115]
- Seychelles[116]
- Sierra Leone[117]
- Singapore[118] (restricted)
- Solomon Islands[48]
- Somalia[119]
- South Korea[120][121]
- South Sudan[122]
- Sri Lanka[123]
- Sudan[124][125]
- Syria[57]
- Taiwan[126]
- Tajikistan[127]
- Thailand[128]
- Togo[129]
- Tonga[130][131][132]
- Tunisia[57]
- Turkmenistan[57]
- Tuvalu[104]
- Uganda[133]
- Ukraine[134]
- United Arab Emirates[48]
- United Kingdom[135][136][137]
- Uruguay[48]
- Uzbekistan[48]
- Vanuatu[57]
- Vietnam[138]
- Yemen[139]
- Zambia[140][141][142]
- Zimbabwe[57]
CARPHA countries and entities[143]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada
- Guyana
- Haiti
- Jamaica
- Saint Kitts and Nevis
- Saint Lucia
- Saint Vincent and the Grenadines
- Suriname[144]
- Trinidad and Tobago
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
- Cook Islands[48]
- Falkland Islands[48]
- French Polynesia[48]
- Greenland[145]
- Guadeloupe[57]
- Guernsey[48]
- Isle of Man[48]
- Jersey[48]
- Northern Cyprus[146]
- Pitcairn[57]
- Saint Helena, Ascension and Tristan da Cunha[57]
- Wallis and Futuna[48]
- World Health Organization[147][2][148][149][150][151]
- Travel-only
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
Pfizer–BioNTech
| |||
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty,[37] is an mRNA vaccine[156] produced by the German company BioNTech and the American company Pfizer.[156][157][158] In Hong Kong, Macau, and Taiwan, Comirnaty is distributed by Fosun Pharma.[159][160][161][162][163][164]
Original
- Full (39)
- Australia[165][166]
- Brazil[167]
- Canada[168][169]
- Marshall Islands[a][b]
- Micronesia[a][b]
- New Zealand[170]
- Palau[a][b]
- Saudi Arabia[173][145]
- Switzerland[174][175]
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Emergency (145)
- Afghanistan[48]
- Albania[178]
- Algeria[179]
- Andorra[180]
- Argentina[181]
- Armenia[182]
- Azerbaijan[57]
- Bahrain[183][184]
- Bangladesh[185]
- Benin[57]
- Bhutan[57]
- Bolivia[48]
- Bosnia and Herzegovina[54]
- Botswana[186]
- Brunei[56]
- Cambodia[57]
- Cameroon[57]
- Cape Verde[48]
- Chile[187]
- China (For German citizens)[188]
- Colombia[189]
- Congo-Kinshasa[190]
- Costa Rica[191]
- Djibouti[57]
- Dominican Republic[192]
- East Timor[57]
- Ecuador[193]
- Egypt[57] Zdroj:https://en.wikipedia.org?pojem=List_of_COVID-19_vaccine_authorizations
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk