A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
Petroleum, also known as crude oil or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons,[1] and is found in geological formations. The name petroleum covers both naturally occurring unprocessed crude oil and petroleum products that consist of refined crude oil.
Petroleum is primarily recovered by oil drilling. Drilling is carried out after studies of structural geology, sedimentary basin analysis, and reservoir characterisation. Unconventional reserves such as oil sands and oil shale exist.
Once extracted, oil is refined and separated, most easily by distillation, into innumerable products for direct use or use in manufacturing. Products include fuels such as petrol (gasoline), diesel, kerosene and jet fuel; asphalt and lubricants; chemical reagents used to make plastics; solvents, textiles, refrigerants, paint, synthetic rubber, fertilizers, pesticides, pharmaceuticals, and thousands of others. Petroleum is used in manufacturing a vast variety of materials essential for modern life,[2] and it is estimated that the world consumes about 100 million barrels (16 million cubic metres) each day. Petroleum production can be extremely profitable and was critical to global economic development in the 20th century, with some countries, so-called "oil states", gaining significant economic and international power because of their control of oil production.
Petroleum exploitation can be damaging to the environment and human health. Extraction, refining and burning of petroleum fuels all release large quantities of greenhouse gases, so petroleum is one of the major contributors to climate change. Other negative environmental effects include direct releases, such as oil spills, and as well as air and water pollution at almost all stages of use. These environmental effects have direct and indirect health consequences for humans. Oil has also been a source of internal and inter-state conflict, leading to both state-led wars and other resource conflicts. Production of petroleum is estimated to reach peak oil before 2035[3] as global economies lower dependencies on petroleum as part of climate change mitigation and a transition towards renewable energy and electrification.[4] Oil has played a key role in industrialization and economic development.[5]
Etymology

The word petroleum comes from Medieval Latin petroleum (literally 'rock oil'), which comes from Latin petra 'rock' (from Greek pétra πέτρα) and oleum 'oil' (from Greek élaion ἔλαιον).[6][7]
The origin of the term stems from monasteries in southern Italy where it was in use by the end of the first millennium as an alternative for the older term "naphtha".[8] After that, the term was used in numerous manuscripts and books, such as in the treatise De Natura Fossilium, published in 1546 by the German mineralogist Georg Bauer, also known as Georgius Agricola.[9] After the advent of the oil industry, during the second half of the 19th century, the term became commonly known for the liquid form of hydrocarbons.
History
Early


Petroleum, in one form or another, has been used since ancient times. More than 4300 years ago, bitumen was mentioned when the Sumerians used it to make boats. A tablet of the legend of the birth of Sargon of Akkad mentions a basket which was closed by straw and bitumen. More than 4000 years ago, according to Herodotus and Diodorus Siculus, asphalt was used in the construction of the walls and towers of Babylon; there were oil pits near Ardericca and Babylon, and a pitch spring on Zakynthos.[10] Great quantities of it were found on the banks of the river Issus, one of the tributaries of the Euphrates. Ancient Persian tablets indicate the medicinal and lighting uses of petroleum in the upper levels of their society.
The use of petroleum in ancient China dates back to more than 2000 years ago. The I Ching, one of the earliest Chinese writings, cites that oil in its raw state, without refining, was first discovered, extracted, and used in China in the first century BCE.[clarification needed] In addition, the Chinese were the first to record the use of petroleum as fuel as early as the fourth century BCE.[11][12][13] By 347 CE, oil was produced from bamboo-drilled wells in China.[14][15]
In the 7th century, petroleum was among the essential ingredients for Greek fire, an incendiary projectile weapon that was used by Byzantine Greeks against Arab ships, which were then attacking Constantinople.[16] Crude oil was also distilled by Persian chemists, with clear descriptions given in Arabic handbooks such as those of Abu Bakr al-Razi (Rhazes).[17] The streets of Baghdad were paved with tar, derived from petroleum that became accessible from natural fields in the region.
In the 9th century, oil fields were exploited in the area around modern Baku, Azerbaijan. These fields were described by the Arab geographer Abu Bakr al-Razi in the 10th century, and by Marco Polo in the 13th century, who described the output of those wells as hundreds of shiploads.[18] Arab and Persian chemists also distilled crude oil to produce flammable products for military purposes. Through Islamic Spain, distillation became available in Western Europe by the 12th century.[19] It has also been present in Romania since the 13th century, being recorded as păcură.[20]
Sophisticated oil pits, 4.5 to 6 metres (15 to 20 ft) deep, were dug by the Seneca people and other Iroquois in Western Pennsylvania as early as 1415–1450. The French General Louis-Joseph de Montcalm encountered Seneca using petroleum for ceremonial fires and as a healing lotion during a visit to Fort Duquesne in 1750.[21]
Early British explorers to Myanmar documented a flourishing oil extraction industry based in Yenangyaung that, in 1795, had hundreds of hand-dug wells under production.[22]
Merkwiller-Pechelbronn is said to be the first European site where petroleum has been explored and used. The still active Erdpechquelle, a spring where petroleum appears mixed with water has been used since 1498, notably for medical purposes.
19th century


The world's first oil well was drilled in 1859 by Edwin Drake at what is now called the Drake Well in Cherrytree Township, Pennsylvania. Drake's well is considered the first because it was drilled, not dug, and used a steam engine. There also was a company associated with it, and it sparked a major oil drilling boom.[23]
Prior to the first well drilled by Drake, there was activity in various parts of the world in the mid-19th century. A group directed by Major Alexeyev of the Bakinskii Corps of Mining Engineers hand-drilled a well in the Baku region of Bibi-Heybat in 1846.[24] There were engine-drilled wells in West Virginia in the same year as Drake's well.[25] An early commercial well was hand dug in Poland in 1853, and another in nearby Romania in 1857. At around the same time the world's first, small, oil refinery was opened at Jasło in Poland, with a larger one opened at Ploiești in Romania shortly after. Romania is the first country in the world to have had its annual crude oil output officially recorded in international statistics: 275 tonnes for 1857.[26][27]
In 1858, Georg Christian Konrad Hunäus found a significant amount of petroleum while drilling for lignite in Wietze, Germany. Wietze later provided about 80% of German consumption in the Wilhelminian Era.[28] The production stopped in 1963, but Wietze has hosted a Petroleum Museum since 1970.[29]
Oil sands have been mined since the 18th century.[30] In Wietze in lower Saxony, natural asphalt/bitumen has been explored since the 18th century.[31] Both in Pechelbronn as in Wietze, the coal industry dominated the petroleum technologies.[32]
Chemist James Young in 1847 noticed a natural petroleum seepage in the coal mine at riddings Alfreton, Derbyshire from which he distilled a light thin oil suitable for use as lamp oil, at the same time obtaining a more viscous oil suitable for lubricating machinery. In 1848, Young set up a small business refining crude oil.[33]
Young eventually succeeded, by distilling cannel coal at low heat, in creating a fluid resembling petroleum, which when treated in the same way as the seep oil gave similar products. Young found that by slow distillation he could obtain several useful liquids from it, one of which he named "paraffine oil" because at low temperatures it congealed into a substance resembling paraffin wax.[33]
The production of these oils and solid paraffin wax from coal formed the subject of his patent dated October 17, 1850. In 1850, Young & Meldrum and Edward William Binney entered into partnership under the title of E.W. Binney & Co. at Bathgate in West Lothian and E. Meldrum & Co. at Glasgow; their works at Bathgate were completed in 1851 and became the first truly commercial oil-works in the world with the first modern oil refinery.[34][clarification needed]
The world's first oil refinery was built in 1856 by Ignacy Łukasiewicz.[35] His achievements also included the discovery of how to distill kerosene from seep oil, the invention of the modern kerosene lamp (1853), the introduction of the first modern street lamp in Europe (1853), and the construction of the world's first modern oil "mine" (1854).[36] at Bóbrka, near Krosno (still operational as of 2020).
The demand for petroleum as a fuel for lighting in North America and around the world quickly grew.[37]
The first commercial oil well in Canada became operational in 1858 at Oil Springs, Ontario (then Canada West).[38] Businessman James Miller Williams dug several wells between 1855 and 1858 before discovering a rich reserve of oil four metres below ground.[39][specify] Williams extracted 1.5 million litres of crude oil by 1860, refining much of it into kerosene lamp oil. Williams's well became commercially viable a year before Drake's Pennsylvania operation and could be argued to be the first commercial oil well in North America.[40] The discovery at Oil Springs touched off an oil boom which brought hundreds of speculators and workers to the area. Advances in drilling continued into 1862 when local driller Shaw reached a depth of 62 metres using the spring-pole drilling method.[41] On January 16, 1862, after an explosion of natural gas, Canada's first oil gusher came into production, shooting into the air at a recorded rate of 480 cubic metres (3,000 bbl) per day.[42] By the end of the 19th century the Russian Empire, particularly the Branobel company in Azerbaijan, had taken the lead in production.[43]
20th century
Access to oil was and still is a major factor in several military conflicts of the 20th century, including World War II, during which oil facilities were a major strategic asset and were extensively bombed.[44] The German invasion of the Soviet Union included the goal to capture the Baku oilfields, as it would provide much-needed oil supplies for the German military which was suffering from blockades.[45]
Oil exploration in North America during the early 20th century later led to the U.S. becoming the leading producer by mid-century. As petroleum production in the U.S. peaked during the 1960s, the United States was surpassed by Saudi Arabia and the Soviet Union in total output.[46][47][48]
In 1973, Saudi Arabia and other Arab nations imposed an oil embargo against the United States, United Kingdom, Japan and other Western nations which supported Israel in the Yom Kippur War of October 1973.[49] The embargo caused an oil crisis. This was followed by the 1979 oil crisis, which was caused by a drop in oil production in the wake of the Iranian Revolution and caused oil prices to more than double.
21st century
The two oil price shocks had many short- and long-term effects on global politics and the global economy.[50] They led to sustained reductions in demand as a result of substitution to other fuels, especially coal and nuclear, and improvements in energy efficiency, facilitated by government policies.[51] High oil prices also induced investment in oil production by non-OPEC countries, including Prudhoe Bay in Alaska, the North Sea offshore fields of the United Kingdom and Norway, the Cantarell offshore field of Mexico, and oil sands in Canada.[52]
About 90 percent of vehicular fuel needs are met by oil. Petroleum also makes up 40 percent of total energy consumption in the United States, but is responsible for only one percent of electricity generation.[53] Petroleum's worth as a portable, dense energy source powering the vast majority of vehicles and as the base of many industrial chemicals makes it one of the world's most important commodities.
The top three oil-producing countries as of 2018 are the United States, Russia, and Saudi Arabia.[54] In 2018, due in part to developments in hydraulic fracturing and horizontal drilling, the United States became the world's largest producer.[55]
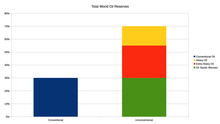
About 80 percent of the world's readily accessible reserves are located in the Middle East, with 62.5 percent coming from the Arab five: Saudi Arabia, United Arab Emirates, Iraq, Qatar, and Kuwait. A large portion of the world's total oil exists as unconventional sources, such as bitumen in Athabasca oil sands and extra heavy oil in the Orinoco Belt. While significant volumes of oil are extracted from oil sands, particularly in Canada, logistical and technical hurdles remain, as oil extraction requires large amounts of heat and water, making its net energy content quite low relative to conventional crude oil. Thus, Canada's oil sands are not expected to provide more than a few million barrels per day in the foreseeable future.[56][57][58]
Composition
Petroleum includes not only crude oil, but all liquid, gaseous and solid hydrocarbons.[clarification needed] Under surface pressure and temperature conditions, lighter hydrocarbons methane, ethane, propane and butane exist as gases, while pentane and heavier hydrocarbons are in the form of liquids or solids. However, in an underground oil reservoir the proportions of gas, liquid, and solid depend on subsurface conditions and on the phase diagram of the petroleum mixture.[59] Some of the components of oil will mix with water: the water associated fraction of the oil.
An oil well produces predominantly crude oil, with some natural gas dissolved in it. Because the pressure is lower at the surface than underground, some of the gas will come out of solution and be recovered (or burned) as associated gas or solution gas. A gas well produces predominantly natural gas. However, because the underground temperature is higher than at the surface, the gas may contain heavier hydrocarbons such as pentane, hexane, and heptane ("natural-gas condensate", often shortened to condensate.) Condensate resembles gasoline in appearance and is similar in composition to some volatile light crude oils.[60][61]
The proportion of light hydrocarbons in the petroleum mixture varies among different oil fields, ranging from as much as 97 percent by weight in the lighter oils to as little as 50 percent in the heavier oils and bitumens.[citation needed]
The hydrocarbons in crude oil are mostly alkanes, cycloalkanes and various aromatic hydrocarbons, while the other organic compounds contain nitrogen, oxygen and sulfur, and trace amounts of metals such as iron, nickel, copper and vanadium. Many oil reservoirs contain live bacteria.[62] The exact molecular composition of crude oil varies widely from formation to formation but the proportion of chemical elements varies over fairly narrow limits as follows:[63]
| Element | Percent range |
|---|---|
| Carbon | 83 to 85% |
| Hydrogen | 10 to 14% |
| Nitrogen | 0.1 to 2% |
| Oxygen | 0.05 to 1.5% |
| Sulfur | 0.05 to 6.0% |
| Metals | < 0.1% |
Four different types of hydrocarbon appear in crude oil. The relative percentage of each varies from oil to oil, determining the properties of each oil.[59]
| Hydrocarbon | Average | Range |
|---|---|---|
| Alkanes (paraffins) | 30% | 15 to 60% |
| Naphthenes | 49% | 30 to 60% |
| Aromatics | 15% | 3 to 30% |
| Asphaltics | 6% | remainder |

The alkanes from pentane (C5H12) to octane (C8H18) are refined into gasoline, the ones from nonane (C9H20) to hexadecane (C16H34) into diesel fuel, kerosene and jet fuel. Alkanes with more than 16 carbon atoms can be refined into fuel oil and lubricating oil. At the heavier end of the range, paraffin wax is an alkane with approximately 25 carbon atoms, while asphalt has 35 and up, although these are usually cracked by modern refineries into more valuable products. The shortest molecules, those with four or fewer carbon atoms, are in a gaseous state at room temperature. They are the petroleum gases. Depending on demand and the cost of recovery, these gases are either flared off, sold as liquefied petroleum gas under pressure, or used to power the refinery's own burners. During the winter, butane (C4H10), is blended into the gasoline pool at high rates, because its high vapour pressure assists with cold starts. Liquified under pressure slightly above atmospheric, it is best known for powering cigarette lighters, but it is also a main fuel source for many developing countries. Propane can be liquified under modest pressure, and is consumed for just about every application relying on petroleum for energy, from cooking to heating to transportation.
The aromatic hydrocarbons are unsaturated hydrocarbons that have one or more benzene rings. They tend to burn with a sooty flame, and many have a sweet aroma. Some are carcinogenic.
These different molecules are separated by fractional distillation at an oil refinery to produce gasoline, jet fuel, kerosene, and other hydrocarbon fractions.
The number of various molecules in an oil sample can be determined by gas chromatography and mass spectrometry.[65] Due to the large number of co-eluted hydrocarbons within oil, many cannot be resolved by traditional gas chromatography and typically appear as a hump in the chromatogram. This unresolved complex mixture (UCM) of hydrocarbons is particularly apparent when analysing weathered oils and extracts from tissues of organisms exposed to oil.
Crude oil varies greatly in appearance depending on its composition. It is usually black or dark brown (although it may be yellowish, reddish, or even greenish). In the reservoir it is usually found in association with natural gas, which being lighter forms a "gas cap" over the petroleum, and saline water which, being heavier than most forms of crude oil, generally sinks beneath it. Crude oil may also be found in a semi-solid form mixed with sand and water, as in the Athabasca oil sands in Canada, where it is usually referred to as crude bitumen. In Canada, bitumen is considered a sticky, black, tar-like form of crude oil which is so thick and heavy that it must be heated or diluted before it will flow.[66] Venezuela also has large amounts of oil in the Orinoco oil sands, although the hydrocarbons trapped in them are more fluid than in Canada and are usually called extra heavy oil. These oil sands resources are called unconventional oil to distinguish them from oil which can be extracted using traditional oil well methods. Between them, Canada and Venezuela contain an estimated 3.6 trillion barrels (570×109 m3) of bitumen and extra-heavy oil, about twice the volume of the world's reserves of conventional oil.[67]
Formation
Fossil petroleum
Petroleum is a fossil fuel derived from fossilized organic materials, such as zooplankton and algae.[70][71] Vast amounts of these remains settled to sea or lake bottoms where they were covered in stagnant water (water with no dissolved oxygen) or sediments such as mud and silt faster than they could decompose aerobically. Approximately 1 m below this sediment, water oxygen concentration was low, below 0.1 mg/L, and anoxic conditions existed. Temperatures also remained constant.[71]
As further layers settled into the sea or lake bed, intense heat and pressure built up in the lower regions. This process caused the organic matter to change, first into a waxy material known as kerogen, found in various oil shales around the world, and then with more heat into liquid and gaseous hydrocarbons via a process known as catagenesis. Formation of petroleum occurs from hydrocarbon pyrolysis in a variety of mainly endothermic reactions at high temperatures or pressures, or both.[71][72] These phases are described in detail below.
Anaerobic decay
In the absence of plentiful oxygen, aerobic bacteria were prevented from decaying the organic matter after it was buried under a layer of sediment or water. However, anaerobic bacteria were able to reduce sulfates and nitrates among the matter to H2S and N2 respectively by using the matter as a source for other reactants. Due to such anaerobic bacteria, at first, this matter began to break apart mostly via hydrolysis: polysaccharides and proteins were hydrolyzed to simple sugars and amino acids respectively. These were further anaerobically oxidized at an accelerated rate by the enzymes of the bacteria: e.g., amino acids went through oxidative deamination to imino acids, which in turn reacted further to ammonia and α-keto acids. Monosaccharides in turn ultimately decayed to CO2 and methane. The anaerobic decay products of amino acids, monosaccharides, phenols and aldehydes combined into fulvic acids. Fats and waxes were not extensively hydrolyzed under these mild conditions.[71]
Kerogen formation
Some phenolic compounds produced from previous reactions worked as bactericides and the actinomycetales order of bacteria also produced antibiotic compounds (e.g., streptomycin). Thus the action of anaerobic bacteria ceased at about 10 m below the water or sediment. The mixture at this depth contained fulvic acids, unreacted and partially reacted fats and waxes, slightly modified lignin, resins and other hydrocarbons.[71] As more layers of organic matter settled into the sea or lake bed, intense heat and pressure built up in the lower regions.[72] As a consequence, compounds of this mixture began to combine in poorly understood ways to kerogen. Combination happened in a similar fashion as phenol and formaldehyde molecules react to urea-formaldehyde resins, but kerogen formation occurred in a more complex manner due to a bigger variety of reactants. The total process of kerogen formation from the beginning of anaerobic decay is called diagenesis, a word that means a transformation of materials by dissolution and recombination of their constituents.[71]
Transformation of kerogen into fossil fuels
Kerogen formation continued to a depth of about 1 km from the Earth's surface where temperatures may reach around 50 °C. Kerogen formation represents a halfway point between organic matter and fossil fuels: kerogen can be exposed to oxygen, oxidize and thus be lost, or it could be buried deeper inside the Earth's crust and be subjected to conditions which allow it to slowly transform into fossil fuels like petroleum. The latter happened through catagenesis in which the reactions were mostly radical rearrangements of kerogen. These reactions took thousands to millions of years and no external reactants were involved. Due to the radical nature of these reactions, kerogen reacted towards two classes of products: those with low H/C ratio (anthracene or products similar to it) and those with high H/C ratio (methane or products similar to it); i.e., carbon-rich or hydrogen-rich products. Because catagenesis was closed off from external reactants, the resulting composition of the fuel mixture was dependent on the composition of the kerogen via reaction stoichiometry. Three types of kerogen exist: type I (algal), II (liptinic) and III (humic), which were formed mainly from algae, plankton and woody plants (this term includes trees, shrubs and lianas) respectively.[71]
Catagenesis was pyrolytic despite the fact that it happened at relatively low temperatures (when compared to commercial pyrolysis plants) of 60 to several hundred °C. Pyrolysis was possible because of the long reaction times involved. Heat for catagenesis came from the decomposition of radioactive materials of the crust, especially 40K, 232Th, 235U and 238U. The heat varied with geothermal gradient and was typically 10–30 °C per km of depth from the Earth's surface. Unusual magma intrusions, however, could have created greater localized heating.[71]
Oil window (temperature range)
Geologists often refer to the temperature range in which oil forms as an "oil window".[73][74][71] Below the minimum temperature oil remains trapped in the form of kerogen. Above the maximum temperature the oil is converted to natural gas through the process of thermal cracking. Sometimes, oil formed at extreme depths may migrate and become trapped at a much shallower level. The Athabasca oil sands are one example of this.[71]
Abiogenic petroleum
An alternative mechanism to the one described above was proposed by Russian scientists in the mid-1850s, the hypothesis of abiogenic petroleum origin (petroleum formed by inorganic means), but this is contradicted by geological and geochemical evidence.[75] Abiogenic sources of oil have been found, but never in commercially profitable amounts. "The controversy isn't over whether abiogenic oil reserves exist," said Larry Nation of the American Association of Petroleum Geologists. "The controversy is over how much they contribute to Earth's overall reserves and how much time and effort geologists should devote to seeking them out."[76]
Reservoirs
This section needs additional citations for verification. (October 2016) |

Three conditions must be present for oil reservoirs to form:
- A source rock rich in hydrocarbon material buried deeply enough for subterranean heat to cook it into oil,
- A porous and permeable reservoir rock where it can accumulate,
- A caprock (seal) or other mechanism to prevent the oil from escaping to the surface. Within these reservoirs, fluids will typically organize themselves like a three-layer cake with a layer of water below the oil layer and a layer of gas above it, although the different layers vary in size between reservoirs. Because most hydrocarbons are less dense than rock or water, they often migrate upward through adjacent rock layers until either reaching the surface or becoming trapped within porous rocks (known as reservoirs) by impermeable rocks above. However, the process is influenced by underground water flows, causing oil to migrate hundreds of kilometres horizontally or even short distances downward before becoming trapped in a reservoir. When hydrocarbons are concentrated in a trap, an oil field forms, from which the liquid can be extracted by drilling and pumping.
The reactions that produce oil and natural gas are often modeled as first order breakdown reactions, where hydrocarbons are broken down to oil and natural gas by a set of parallel reactions, and oil eventually breaks down to natural gas by another set of reactions. The latter set is regularly used in petrochemical plants and oil refineries.
Petroleum has mostly been recovered by oil drilling (natural petroleum springs are rare). Drilling is carried out after studies of structural geology (at the reservoir scale), sedimentary basin analysis, and reservoir characterisation (mainly in terms of the porosity and permeability of geologic reservoir structures).[77][78] Wells are drilled into oil reservoirs to extract the crude oil. "Natural lift" production methods that rely on the natural reservoir pressure to force the oil to the surface are usually sufficient for a while after reservoirs are first tapped. In some reservoirs, such as in the Middle East, the natural pressure is sufficient over a long time. The natural pressure in most reservoirs, however, eventually dissipates. Then the oil must be extracted using "artificial lift" means. Over time, these "primary" methods become less effective and "secondary" production methods may be used. A common secondary method is "waterflood" or injection of water into the reservoir to increase pressure and force the oil to the drilled shaft or "wellbore." Eventually "tertiary" or "enhanced" oil recovery methods may be used to increase the oil's flow characteristics by injecting steam, carbon dioxide and other gases or chemicals into the reservoir. In the United States, primary production methods account for less than 40 percent of the oil produced on a daily basis, secondary methods account for about half, and tertiary recovery the remaining 10 percent. Extracting oil (or "bitumen") from oil/tar sand and oil shale deposits requires mining the sand or shale and heating it in a vessel or retort, or using "in-situ" methods of injecting heated liquids into the deposit and then pumping the liquid back out saturated with oil.
Unconventional oil reservoirs
Oil-eating bacteria biodegrade oil that has escaped to the surface. Oil sands are reservoirs of partially biodegraded oil still in the process of escaping and being biodegraded, but they contain so much migrating oil that, although most of it has escaped, vast amounts are still present—more than can be found in conventional oil reservoirs. The lighter fractions of the crude oil are destroyed first, resulting in reservoirs containing an extremely heavy form of crude oil, called crude bitumen in Canada, or extra-heavy crude oil in Venezuela. These two countries have the world's largest deposits of oil sands.[79]
On the other hand, oil shales are source rocks that have not been exposed to heat or pressure long enough to convert their trapped hydrocarbons into crude oil. Technically speaking, oil shales are not always shales and do not contain oil, but are fined-grain sedimentary rocks containing an insoluble organic solid called kerogen. The kerogen in the rock can be converted into crude oil using heat and pressure to simulate natural processes. The method has been known for centuries and was patented in 1694 under British Crown Patent No. 330 covering, "A way to extract and make great quantities of pitch, tar, and oil out of a sort of stone." Although oil shales are found in many countries, the United States has the world's largest deposits.[80]
Classification
The examples and perspective in this article may not represent a worldwide view of the subject. (January 2024) |

The petroleum industry generally classifies crude oil by the geographic location it is produced in (e.g., West Texas Intermediate, Brent, or Oman), its API gravity (an oil industry measure of density), and its sulfur content. Crude oil may be considered light if it has low density, heavy if it has high density, or medium if it has a density between that of light and heavy.[81] Additionally, it may be referred to as sweet if it contains relatively little sulfur or sour if it contains substantial amounts of sulfur.[82]
The geographic location is important because it affects transportation costs to the refinery. Light crude oil is more desirable than heavy oil since it produces a higher yield of gasoline, while sweet oil commands a higher price than sour oil because it has fewer environmental problems and requires less refining to meet sulfur standards imposed on fuels in consuming countries. Each crude oil has unique molecular characteristics which are revealed by the use of crude oil assay analysis in petroleum laboratories.[83]
Barrels from an area in which the crude oil's molecular characteristics have been determined and the oil has been classified are used as pricing references throughout the world. Some of the common reference crudes are:[citation needed]
- West Texas Intermediate (WTI), a very high-quality, sweet, light oil delivered at Cushing, Oklahoma for North American oil
- Brent Blend, consisting of 15 oils from fields in the Brent and Ninian systems in the East Shetland Basin of the North Sea. The oil landed at Sullom Voe terminal in Shetland. Oil production from Europe, Africa and Middle Eastern oil flowing West tends to be priced off this oil, which forms a benchmark
- Dubai-Oman, used as a benchmark for the Middle East sour crude oil flowing to the Asia-Pacific region
- Tapis (from Malaysia, used as a reference for light Far East oil)
- Minas (from Indonesia, used as a reference for heavy Far East oil)
- The OPEC Reference Basket, a weighted average of oil blends from various OPEC (Organization of the Petroleum Exporting Countries) countries
- Midway Sunset Heavy, by which heavy oil in California is priced[84][failed verification]
- Western Canadian Select the benchmark crude oil for emerging heavy, high TAN (acidic) crudes.[85]
There are declining amounts of these benchmark oils being produced each year, so other oils are more commonly what is actually delivered. While the reference price may be for West Texas Intermediate delivered at Cushing, the actual oil being traded may be a discounted Canadian heavy oil – Western Canadian Select – delivered at Hardisty, Alberta, and for a Brent Blend delivered at Shetland, it may be a discounted Russian Export Blend delivered at the port of Primorsk.[86]
Once extracted, oil is refined and separated, most easily by distillation, into numerous products for direct use or use in manufacturing, such as petrol (gasoline), diesel and kerosene to asphalt and chemical reagents (ethylene, propylene, butene, acrylic acid, para-xylene[87]) used to make plastics, pesticides and pharmaceuticals.[88]
Use
In terms of volume, most petroleum is converted into fuels for combustion engines. In terms of value, petroleum underpins the petrochemical industry, which includes many high value products such as pharmaceuticals and plastics.
Fuels and lubricants
Petroleum is used mostly, by volume, for refining into fuel oil and gasoline, both important primary energy sources. 84% by volume of the hydrocarbons present in petroleum is converted into fuels, including gasoline, diesel, jet, heating, and other fuel oils, and liquefied petroleum gas.[89]
Due to its high energy density, easy transportability and relative abundance, oil has become the world's most important source of energy since the mid-1950s. Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics; the 16 percent not used for energy production is converted into these other materials. Petroleum is found in porous rock formations in the upper strata of some areas of the Earth's crust. There is also petroleum in oil sands (tar sands). Known oil reserves are typically estimated at 190 km3 (1.2 trillion (short scale) barrels) without oil sands,[90] or 595 km3 (3.74 trillion barrels) with oil sands.[91] Consumption is currently around 84 million barrels (13.4×106 m3) per day, or 4.9 km3 per year, yielding a remaining oil supply of only about 120 years, if current demand remains static.[92] More recent studies, however, put the number at around 50 years.[93][94]
Closely related to fuels for combustion engines are Lubricants, greases, and viscosity stabilizers. All are derived from petroleum.
Chemicals
All pharmaceuticals are derived from petroleum, albeit via mutlistep processes.[citation needed] Modern medicine depends on petroleum as a source of building blocks, reagents, and solvents.[95] Similarly, virtually all pesticides - insecticides, herbicides, etc. - are derived from petroleum. Pesticides have profoundly affected life expectancies by controlling disease vectors and by increasing yields of crops. Like pharmaceuticals, pesticides are in essence petrochemicals. Virtually all plastics and synthetic polymers are derived from petroleum, which is the source of monomers. Alkenes (olefins) are one important class of these precursor molecules.
Other derivatives

- Wax, used in the packaging of frozen foods, among others, Paraffin wax, derived from petroleum oil.[96]
- Sulfur and its derivative sulfuric acid. Hydrogen sulfide is a product of sulfur removal from petroleum fraction. It is oxidized to elemental sulfur and then to sulfuric acid.
- Bulk tar and Asphalt
- Petroleum coke, used in speciality carbon products or as solid fuel
Industry

The petroleum industry, also known as the oil industry or the oil patch, includes the global processes of exploration, extraction, refining, transportation (often by oil tankers and pipelines), and marketing of petroleum products. The largest volume products of the industry are fuel oil and gasoline (petrol). Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, synthetic fragrances, and plastics. The industry is usually divided into three major components: upstream, midstream, and downstream. Upstream regards exploration and extraction of crude oil, midstream encompasses transportation and storage of crude, and downstream concerns refining crude oil into various end products.
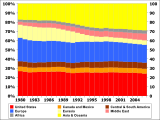
Petroleum is vital to many industries, and is necessary for the maintenance of industrial civilization in its current configuration, making it a critical concern for many nations. Oil accounts for a large percentage of the world's energy consumption, ranging from a low of 32% for Europe and Asia, to a high of 53% for the Middle East.
Other geographic regions' consumption patterns are as follows: South and Central America (44%), Africa (41%), and North America (40%). The world consumes 36 billion barrels (5.8 km3) of oil per year,[97] with developed nations being the largest consumers. The United States consumed 18% of the oil produced in 2015.[98] The production, distribution, refining, and retailing of petroleum taken as a whole represents the world's largest industry in terms of dollar value.Transport

In the 1950s, shipping costs made up 33 percent of the price of oil transported from the Persian Gulf to the United States,[100] but due to the development of supertankers in the 1970s, the cost of shipping dropped to only 5 percent of the price of Persian oil in the US.[100] Due to the increase in the value of crude oil during the last 30 years, the share of the shipping cost on the final cost of the delivered commodity was less than 3% in 2010.
Priceedit
This article needs to be updated. (March 2022) |



The price of oil, or the oil price, generally refers to the spot price of a barrel (159 litres) of benchmark crude oil—a reference price for buyers and sellers of crude oil such as West Texas Intermediate (WTI), Brent Crude, Dubai Crude, OPEC Reference Basket, Tapis crude, Bonny Light, Urals oil, Isthmus, and Western Canadian Select (WCS).[101][102] Oil prices are determined by global supply and demand, rather than any country's domestic production level.
The global price of crude oil was relatively consistent in the nineteenth century and early twentieth century.[103] This changed in the 1970s, with a significant increase in the price of oil globally.[103] There have been a number of structural drivers of global oil prices historically, including oil supply, demand, and storage shocks, and shocks to global economic growth affecting oil prices.[104] Notable events driving significant price fluctuations include the 1973 OPEC oil embargo targeting nations that had supported Israel during the Yom Kippur War,[105]: 329 resulting in the 1973 oil crisis, the Iranian Revolution in the 1979 oil crisis, the financial crisis of 2007–2008, and the more recent 2013 oil supply glut that led to the "largest oil price declines in modern history" in 2014 to 2016. The 70% decline in global oil prices was "one of the three biggest declines since World War II, and the longest lasting since the supply-driven collapse of 1986."[106] By 2015, the United States had become the third-largest producer of oil and resumed exporting oil upon repeal of its 40-year export ban.[107][108][109]
The 2020 Russia–Saudi Arabia oil price war resulted in a 65% decline in global oil prices at the beginning of the COVID-19 pandemic.[110][111] In 2021, the record-high energy prices were driven by a global surge in demand as the world recovered from the COVID-19 recession.[112][113][114] By December 2021, an unexpected rebound in the demand for oil from United States, China and India, coupled with U.S. shale industry investors' "demands to hold the line on spending", has contributed to "tight" oil inventories globally.[115] On 18 January 2022, as the price of Brent crude oil reached its highest since 2014—$88, concerns were raised about the rising cost of gasoline—which hit a record high in the United Kingdom.[116]Tradeedit

Crude oil is traded as a future on both the NYMEX and ICE exchanges.[117] Futures contracts are agreements in which buyers and sellers agree to purchase and deliver specific amounts of physical crude oil on a given date in the future. A contract covers any multiple of 1000 barrels and can be purchased up to nine years into the future.[118]
Use by countryedit
Consumption statisticsedit
-
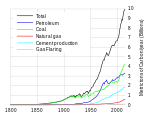
Global fossil carbon emissions, an indicator of consumption, from 1800.TotalOil
-
Rate of world energy usage per year from 1970.[119]
-
Daily oil consumption from 1980 to 2006.
-
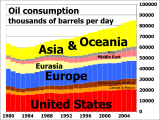
Oil consumption 1980 to 2007 by region.
Consumptionedit
According to the US Energy Information Administration (EIA) estimate for 2017, the world consumes 98.8 million barrels of oil each day.[120]

| > 0.07 0.07–0.05 0.05–0.035 0.035–0.025 0.025–0.02 | 0.02–0.015 0.015–0.01 0.01–0.005 0.005–0.0015 < 0.0015 |
This table orders the amount of petroleum consumed in 2011 in thousand barrels (1000 bbl) per day and in thousand cubic metres (1000 m3) per day:[121][122]
| Consuming nation 2011 | (1000 bbl/ day) |
(1000 m3/ day) |
Population in millions |
bbl/year per capita |
m3/year per capita |
National production/ consumption |
|---|---|---|---|---|---|---|
| United States 1 | 18,835.5 | 2,994.6 | 314 | 21.8 | 3.47 | 0.51 |
| China | 9,790.0 | 1,556.5 | 1345 | 2.7 | 0.43 | 0.41 |
| Japan 2 | 4,464.1 | 709.7 | 127 | 12.8 | 2.04 | 0.03
Zdroj:https://en.wikipedia.org?pojem=Crude_oil Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Analytika
Antropológia Aplikované vedy Bibliometria Dejiny vedy Encyklopédie Filozofia vedy Forenzné vedy Humanitné vedy Knižničná veda Kryogenika Kryptológia Kulturológia Literárna veda Medzidisciplinárne oblasti Metódy kvantitatívnej analýzy Metavedy Metodika Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok. www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk |