A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Hydrogen sulfide[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 3535004 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.070 | ||
| EC Number |
| ||
| 303 | |||
| KEGG | |||
| MeSH | Hydrogen+sulfide | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1053 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H2S | |||
| Molar mass | 34.08 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Foul, pungent, like that of rotten eggs | ||
| Density | 1.539 g.L−1 (0°C)[2] | ||
| Melting point | −85.5[3] °C (−121.9 °F; 187.7 K) | ||
| Boiling point | −59.55[3] °C (−75.19 °F; 213.60 K) | ||
| 3.980 g dm−3 (at 20 °C) [4] | |||
| Vapor pressure | 1740 kPa (at 21 °C) | ||
| Acidity (pKa) | 7.0[5][6] | ||
| Conjugate acid | Sulfonium | ||
| Conjugate base | Bisulfide | ||
| −25.5·10−6 cm3/mol | |||
Refractive index (nD)
|
1.000644 (0 °C)[2] | ||
| Structure | |||
| C2v | |||
| Bent | |||
| 0.97 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
1.003 J K−1 g−1 | ||
Std molar
entropy (S⦵298) |
206 J mol−1 K−1[7] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−21 kJ mol−1[7] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable and highly toxic | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H220, H330, H400 | |||
| P210, P260, P271, P273, P284, P304+P340, P310, P320, P377, P381, P391, P403, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −82.4 °C (−116.3 °F; 190.8 K)[10] | ||
| 232 °C (450 °F; 505 K) | |||
| Explosive limits | 4.3–46% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
| ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
C 20 ppm; 50 ppm [8] | ||
REL (Recommended)
|
C 10 ppm (15 mg/m3) [8] | ||
IDLH (Immediate danger)
|
100 ppm[8] | ||
| Related compounds | |||
Related hydrogen chalcogenides
|
|||
Related compounds
|
Phosphine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Hydrogen sulfide is a chemical compound with the formula H2S. It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs.[11] Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777.[12]
Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts,[clarification needed] damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death.[13][14] Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule.[15]
Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestion, which is done by sulfate-reducing microorganisms. It also occurs in volcanic gases, natural gas deposits, and sometimes in well-drawn water.
Properties
Hydrogen sulfide is slightly denser than air. A mixture of H2S and air can be explosive.
Oxidation
In general, hydrogen sulfide acts as a reducing agent, as indicated by its ability to reduce sulfur dioxide in the Claus process. Hydrogen sulfide burns in oxygen with a blue flame to form sulfur dioxide (SO2) and water:
- 2 H2S + 3 O2 → 2 SO2 + 2 H2O
If an excess of oxygen is present, sulfur trioxide (SO3) is formed, which quickly hydrates to sulfuric acid:
- H2S + 2 O2 → H2SO4
Acid-base properties
It is slightly soluble in water and acts as a weak acid (pKa = 6.9 in 0.01–0.1 mol/litre solutions at 18 °C), giving the hydrosulfide ion HS−. Hydrogen sulfide and its solutions are colorless. When exposed to air, it slowly oxidizes to form elemental sulfur, which is not soluble in water. The sulfide anion S2− is not formed in aqueous solution.[16]
Extreme temperatures and pressures
At pressures above 90 GPa (gigapascal), hydrogen sulfide becomes a metallic conductor of electricity. When cooled below a critical temperature this high-pressure phase exhibits superconductivity. The critical temperature increases with pressure, ranging from 23 K at 100 GPa to 150 K at 200 GPa.[17] If hydrogen sulfide is pressurized at higher temperatures, then cooled, the critical temperature reaches 203 K (−70 °C), the highest accepted superconducting critical temperature as of 2015. By substituting a small part of sulfur with phosphorus and using even higher pressures, it has been predicted that it may be possible to raise the critical temperature to above 0 °C (273 K) and achieve room-temperature superconductivity.[18]
Hydrogen sulfide decomposes without a presence of a catalyst under atmospheric pressure around 1200 °C into hydrogen and sulfur.[19]
Tarnishing
Hydrogen sulfide reacts with metal ions to form metal sulfides, which are insoluble, often dark colored solids. Lead(II) acetate paper is used to detect hydrogen sulfide because it readily converts to lead(II) sulfide, which is black. Treating metal sulfides with strong acid or electrolysis often liberates hydrogen sulfide. Hydrogen sulfide is also responsible for tarnishing on various metals including copper and silver; the chemical responsible for black toning found on silver coins is silver sulfide (Ag2S), which is produced when the silver on the surface of the coin reacts with atmospheric hydrogen sulfide.[20] Coins that have been subject to toning by hydrogen sulfide and other sulfur-containing compounds may have the toning add to the numismatic value of a coin based on aesthetics, as the toning may produce thin-film interference, resulting in the coin taking on an attractive coloration.[21] Coins can also be intentionally treated with hydrogen sulfide to induce toning, though artificial toning can be distinguished from natural toning, and is generally criticised among collectors.[22]
Production
Hydrogen sulfide is most commonly obtained by its separation from sour gas, which is natural gas with a high content of H2S. It can also be produced by treating hydrogen with molten elemental sulfur at about 450 °C. Hydrocarbons can serve as a source of hydrogen in this process.[23]
- S + H2 → H2S
The very favorable thermodynamics for the hydrogenation of sulfur implies that the dehydrogenation (or cracking) of hydrogen sulfide would require very high temperatures.[24]
A standard lab preparation is to treat ferrous sulfide with a strong acid in a Kipp generator:
- FeS + 2 HCl → FeCl2 + H2S
For use in qualitative inorganic analysis, thioacetamide is used to generate H2S:
- CH3C(S)NH2 + H2O → CH3C(O)NH2 + H2S
Many metal and nonmetal sulfides, e.g. aluminium sulfide, phosphorus pentasulfide, silicon disulfide liberate hydrogen sulfide upon exposure to water:[25]
- 6 H2O + Al2S3 → 3 H2S + 2 Al(OH)3
This gas is also produced by heating sulfur with solid organic compounds and by reducing sulfurated organic compounds with hydrogen. It can also be produced by mixing ammonium thiocyanate to concentrated sulphuric acid and adding water to it.
Biosynthesis
Hydrogen sulfide can be generated in cells via enzymatic or non-enzymatic pathways. Three enzymes catalyze formation of H
2S: cystathionine γ-lyase (CSE), cystathionine β-synthetase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST).[26] CBS and CSE are the main proponents of H2S biogenesis, which follows the trans-sulfuration pathway.[27] These enzymes have been identified in a breadth of biological cells and tissues, and their activity is induced by a number of disease states.[28] These enzymes are characterized by the transfer of a sulfur atom from methionine to serine to form a cysteine molecule.[27] 3-MST also contributes to hydrogen sulfide production by way of the cysteine catabolic pathway.[28][27] Dietary amino acids, such as methionine and cysteine serve as the primary substrates for the transulfuration pathways and in the production of hydrogen sulfide. Hydrogen sulfide can also be derived from proteins such as ferredoxins and Rieske proteins.[28]
Sulfate-reducing (resp. sulfur-reducing) bacteria generate usable energy under low-oxygen conditions by using sulfates (resp. elemental sulfur) to oxidize organic compounds or hydrogen; this produces hydrogen sulfide as a waste product.
Water heaters can aid the conversion of sulfate in water to hydrogen sulfide gas. This is due to providing a warm environment sustainable for sulfur bacteria and maintaining the reaction which interacts between sulfate in the water and the water heater anode, which is usually made from magnesium metal.[29]
Signalling role
H2S in the body acts as a gaseous signaling molecule with implications for health and in diseases.[26] [30][31]
Hydrogen sulfide is involved in vasodilation in animals, as well as in increasing seed germination and stress responses in plants.[32] Hydrogen sulfide signaling is moderated by reactive oxygen species (ROS) and reactive nitrogen species (RNS).[32] H2S has been shown to interact with NO resulting in several different cellular effects, as well as the formation of another signal called nitrosothiol.[32] Hydrogen sulfide is also known to increase the levels of glutathione, which acts to reduce or disrupt ROS levels in cells.[32]
The field of H2S biology has advanced from environmental toxicology to investigate the roles of endogenously produced H2S in physiological conditions and in various pathophysiological states.[33] H2S has been implicated in cancer and Down syndrome and vascular disease.[34][35][36][37]
It inhibits Complex IV of the mitochondrial electron transport chain, which effectively reduces ATP generation and biochemical activity within cells.[32]
Uses
Production of sulfur
Hydrogen sulfide is mainly consumed as a precursor to elemental sulfur. This conversion, called the Claus process, involves partial oxidation to sulfur dioxide. The latter reacts with hydrogen sulfide to give elemental sulfur. The conversion is catalyzed by alumina.[38]
- 2H2S + SO2→ 3S + 2H2O
Production of thioorganic compounds
Many fundamental organosulfur compounds are produced using hydrogen sulfide. These include methanethiol, ethanethiol, and thioglycolic acid.[23] Hydrosulfides can be used in the production of thiophenol.[39]
Production of metal sulfides
Upon combining with alkali metal bases, hydrogen sulfide converts to alkali hydrosulfides such as sodium hydrosulfide and sodium sulfide:
- H2S + NaOH → NaSH + H2O
- NaSH + NaOH → Na2S + H2O
Sodium sulfides are used in the paper making industry. Specifically, salts of SH− break bonds between lignin and cellulose components of pulp in the Kraft process.[23]
As indicated above, many metal ions react with hydrogen sulfide to give the corresponding metal sulfides. Oxidic ores are sometimes treated with hydrogen sulfide to give the corresponding metal sulfides which are more readily purified byy flotation.[23] Metal parts are sometimes passivated with hydrogen sulfide. Catalysts used in hydrodesulfurization are routinely activated with hydrogen sulfide.
Hydrogen sulfide was a reagent in the qualitative inorganic analysis of metal ions. In these analyses, heavy metal (and nonmetal) ions (e.g., Pb(II), Cu(II), Hg(II), As(III)) are precipitated from solution upon exposure to H2S. The components of the resulting solid are then identified by their reactivity.
Miscellaneous applications
Hydrogen sulfide is used to separate deuterium oxide, or heavy water, from normal water via the Girdler sulfide process.
A suspended animation-like state has been induced in rodents with the use of hydrogen sulfide, resulting in hypothermia with a concomitant reduction in metabolic rate. Oxygen demand was also reduced, thereby protecting against hypoxia. In addition, hydrogen sulfide has been shown to reduce inflammation in various situations.[40]
Occurrence

Volcanoes and some hot springs (as well as cold springs) emit some H2S. Hydrogen sulfide can be present naturally in well water, often as a result of the action of sulfate-reducing bacteria.[41][better source needed] Hydrogen sulfide is produced by the human body in small quantities through bacterial breakdown of proteins containing sulfur in the intestinal tract, therefore it contributes to the characteristic odor of flatulence. It is also produced in the mouth (halitosis).[42]
A portion of global H2S emissions are due to human activity. By far the largest industrial source of H2S is petroleum refineries: The hydrodesulfurization process liberates sulfur from petroleum by the action of hydrogen. The resulting H2S is converted to elemental sulfur by partial combustion via the Claus process, which is a major source of elemental sulfur. Other anthropogenic sources of hydrogen sulfide include coke ovens, paper mills (using the Kraft process), tanneries and sewerage. H2S arises from virtually anywhere where elemental sulfur comes in contact with organic material, especially at high temperatures. Depending on environmental conditions, it is responsible for deterioration of material through the action of some sulfur oxidizing microorganisms. It is called biogenic sulfide corrosion.
In 2011 it was reported that increased concentrations of H2S were observed in the Bakken formation crude, possibly due to oil field practices, and presented challenges such as "health and environmental risks, corrosion of wellbore, added expense with regard to materials handling and pipeline equipment, and additional refinement requirements".[43]
Besides living near gas and oil drilling operations, ordinary citizens can be exposed to hydrogen sulfide by being near waste water treatment facilities, landfills and farms with manure storage. Exposure occurs through breathing contaminated air or drinking contaminated water.[44]
In municipal waste landfill sites, the burial of organic material rapidly leads to the production of anaerobic digestion within the waste mass and, with the humid atmosphere and relatively high temperature that accompanies biodegradation, biogas is produced as soon as the air within the waste mass has been reduced. If there is a source of sulfate bearing material, such as plasterboard or natural gypsum (calcium sulfate dihydrate), under anaerobic conditions sulfate reducing bacteria converts this to hydrogen sulfide. These bacteria cannot survive in air but the moist, warm, anaerobic conditions of buried waste that contains a high source of carbon – in inert landfills, paper and glue used in the fabrication of products such as plasterboard can provide a rich source of carbon[45] – is an excellent environment for the formation of hydrogen sulfide.
In industrial anaerobic digestion processes, such as waste water treatment or the digestion of organic waste from agriculture, hydrogen sulfide can be formed from the reduction of sulfate and the degradation of amino acids and proteins within organic compounds.[46] Sulfates are relatively non-inhibitory to methane forming bacteria but can be reduced to H2S by sulfate reducing bacteria, of which there are several genera.[47]
Removal from water
A number of processes have been designed to remove hydrogen sulfide from drinking water.[48]
- Continuous chlorination
- For levels up to 75 mg/L chlorine is used in the purification process as an oxidizing chemical to react with hydrogen sulfide. This reaction yields insoluble solid sulfur. Usually the chlorine used is in the form of sodium hypochlorite.[49]
- Aeration
- For concentrations of hydrogen sulfide less than 2 mg/L aeration is an ideal treatment process. Oxygen is added to water and a reaction between oxygen and hydrogen sulfide react to produce odorless sulfate.[50]
- Nitrate addition
- Calcium nitrate can be used to prevent hydrogen sulfide formation in wastewater streams.
Removal from fuel gases
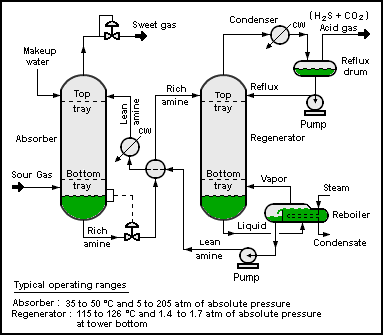
Hydrogen sulfide is commonly found in raw natural gas and biogas. It is typically removed by amine gas treating technologies. In such processes, the hydrogen sulfide is first converted to an ammonium salt, whereas the natural gas is unaffected.
- RNH2 + H2S ⇌ [RNH3+ + SH−
The bisulfide anion is subsequently regenerated by heating of the amine sulfide solution. Hydrogen sulfide generated in this process is typically converted to elemental sulfur using the Claus Process.
Safety
The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is stinkdamp. Hydrogen sulfide is a highly toxic and flammable gas (flammable range: 4.3–46%). It can poison several systems in the body, although the nervous system is most affected.[citation needed] The toxicity of H2S is comparable with that of carbon monoxide.[51] It binds with iron in the mitochondrial cytochrome enzymes, thus preventing cellular respiration. Its toxic properties were described in detail in 1843 by Justus von Liebig.[52]
Even before hydrogen sulfide was discovered, Italian physician Bernardino Ramazzini hypothesized in his 1713 book De Morbis Artificum Diatriba that occupational diseases of sewer-workers and blackening of coins in their clothes may be caused by an unknown invisible volatile acid (moreover, in late 18th century toxic gas emanation from Paris sewers became a problem for the citizens and authorities).[53]
Although very pungent at first (it smells like rotten eggs[54]), it quickly deadens the sense of smell, creating temporary anosmia,[55] so victims may be unaware of its presence until it is too late. Safe handling procedures are provided by its safety data sheet (SDS).[56]
Low-level exposure
Since hydrogen sulfide occurs naturally in the body, the environment, and the gut, enzymes exist to metabolize it. At some threshold level, believed to average around 300–350 ppm, the oxidative enzymes become overwhelmed. Many personal safety gas detectors, such as those used by utility, sewage and petrochemical workers, are set to alarm at as low as 5 to 10 ppm and to go into high alarm at 15 ppm. Metabolism causes oxidation to sulfate, which is harmless.[57] Hence, low levels of hydrogen sulfide may be tolerated indefinitely.
Exposure to lower concentrations can result in eye irritation, a sore throat and cough, nausea, shortness of breath, and fluid in the lungs.[51] These effects are believed to be due to hydrogen sulfide combining with alkali present in moist surface tissues to form sodium sulfide, a caustic.[58] These symptoms usually subside in a few weeks.
Long-term, low-level exposure may result in fatigue, loss of appetite, headaches, irritability, poor memory, and dizziness. Chronic exposure to low level H2S (around 2 ppm) has been implicated in increased miscarriage and reproductive health issues among Russian and Finnish wood pulp workers,[59] but the reports have not (as of 1995) been replicated.
High-level exposure
Short-term, high-level exposure can induce immediate collapse, with loss of breathing and a high probability of death. If death does not occur, high exposure to hydrogen sulfide can lead to cortical pseudolaminar necrosis, degeneration of the basal ganglia and cerebral edema.[51] Although respiratory paralysis may be immediate, it can also be delayed up to 72 hours.[60] Diagnostic of extreme poisoning by H2S is the discolouration of copper coins in the pockets of the victim.
Inhalation of H2S resulted in about 7 workplace deaths per year in the U.S. (2011–2017 data), second only to carbon monoxide (17 deaths per year) for workplace chemical inhalation deaths.[61]
Zdroj:https://en.wikipedia.org?pojem=Hydrogen_sulfide
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk