
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
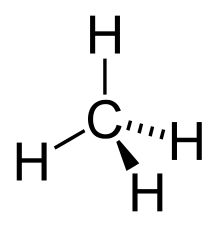
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single.[1] Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane (CH4), where n = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like pentacontane (C50H102) or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (C14H30).
The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula CnH2n+2, and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote any saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or polycyclic, despite their having a distinct general formula (i.e. cycloalkanes are CnH2n).
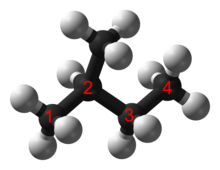
In an alkane, each carbon atom is sp3-hybridized with 4 sigma bonds (either C–C or C–H), and each hydrogen atom is joined to one of the carbon atoms (in a C–H bond). The longest series of linked carbon atoms in a molecule is known as its carbon skeleton or carbon backbone. The number of carbon atoms may be considered as the size of the alkane.
One group of the higher alkanes are waxes, solids at standard ambient temperature and pressure (SATP), for which the number of carbon atoms in the carbon backbone is greater than about 17. With their repeated –CH2 units, the alkanes constitute a homologous series of organic compounds in which the members differ in molecular mass by multiples of 14.03 u (the total mass of each such methylene-bridge unit, which comprises a single carbon atom of mass 12.01 u and two hydrogen atoms of mass ~1.01 u each).
Methane is produced by methanogenic bacteria and some long-chain alkanes function as pheromones in certain animal species or as protective waxes in plants and fungi. Nevertheless, most alkanes do not have much biological activity. They can be viewed as molecular trees upon which can be hung the more active/reactive functional groups of biological molecules.
The alkanes have two main commercial sources: petroleum (crude oil) and natural gas.
An alkyl group is an alkane-based molecular fragment that bears one open valence for bonding. They are generally abbreviated with the symbol for any organyl group, R, although Alk is sometimes used to specifically symbolize an alkyl group (as opposed to an alkenyl group or aryl group).
Structure and classification
Ordinarily the C-C single bond distance is 1.53 ångströms (1.53×10−10 m).[2] Saturated hydrocarbons can be linear, branched, or cyclic. The third group is sometimes called cycloalkanes.[1] Very complicated structures are possible by combining linear, branch, cyclic alkanes.
Isomerism

Alkanes with more than three carbon atoms can be arranged in various ways, forming structural isomers. The simplest isomer of an alkane is the one in which the carbon atoms are arranged in a single chain with no branches. This isomer is sometimes called the n-isomer (n for "normal", although it is not necessarily the most common). However, the chain of carbon atoms may also be branched at one or more points. The number of possible isomers increases rapidly with the number of carbon atoms. For example, for acyclic alkanes:[3]
- C1: methane only
- C2: ethane only
- C3: propane only
- C4: 2 isomers: butane and isobutane
- C5: 3 isomers: pentane, isopentane, and neopentane
- C6: 5 isomers: hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane
- C7: 9 isomers: heptane, 2-methylhexane, 3-methylhexane, 2,2-dimethylpentane, 2,3-dimethylpentane, 2,4-dimethylpentane, 3,3-dimethylpentane, 3-ethylpentane, 2,2,3-trimethylbutane
- C8: 18 isomers: octane, 2-methylheptane, 3-methylheptane, 4-methylheptane, 2,2-dimethylhexane, 2,3-dimethylhexane, 2,4-dimethylhexane, 2,5-dimethylhexane, 3,3-dimethylhexane, 3,4-dimethylhexane, 3-ethylhexane, 2,2,3-trimethylpentane, 2,2,4-trimethylpentane, 2,3,3-trimethylpentane, 2,3,4-trimethylpentane, 3-ethyl-2-methylpentane, 3-ethyl-3-methylpentane, 2,2,3,3-tetramethylbutane
- C9: 35 isomers
- C10: 75 isomers
- C12: 355 isomers
- C32: 27,711,253,769 isomers
- C60: 22,158,734,535,770,411,074,184 isomers, many of which are not stable
Branched alkanes can be chiral. For example, 3-methylhexane and its higher homologues are chiral due to their stereogenic center at carbon atom number 3. The above list only includes differences of connectivity, not stereochemistry. In addition to the alkane isomers, the chain of carbon atoms may form one or more rings. Such compounds are called cycloalkanes, and are also excluded from the above list because changing the number of rings changes the molecular formula. For example, cyclobutane and methylcyclopropane are isomers of each other (C4H8), but are not isomers of butane (C4H10).
Branched alkanes are more thermodynamically stable than their linear (or less branched) isomers. For example, the highly branched 2,2,3,3-tetramethylbutane is about 1.9 kcal/mol more stable than its linear isomer, n-octane.[4]
Nomenclature
The IUPAC nomenclature (systematic way of naming compounds) for alkanes is based on identifying hydrocarbon chains. Unbranched, saturated hydrocarbon chains are named systematically with a Greek numerical prefix denoting the number of carbons and the suffix "-ane".[5]
In 1866, August Wilhelm von Hofmann suggested systematizing nomenclature by using the whole sequence of vowels a, e, i, o and u to create suffixes -ane, -ene, -ine (or -yne), -one, -une, for the hydrocarbons CnH2n+2, CnH2n, CnH2n−2, CnH2n−4, CnH2n−6.[6] In modern nomenclature, the first three specifically name hydrocarbons with single, double and triple bonds;[7] while "-one" now represents a ketone.
Linear alkanes
Straight-chain alkanes are sometimes indicated by the prefix "n-" or "n-"(for "normal") where a non-linear isomer exists. Although this is not strictly necessary and is not part of the IUPAC naming system, the usage is still common in cases where one wishes to emphasize or distinguish between the straight-chain and branched-chain isomers, e.g., "n-butane" rather than simply "butane" to differentiate it from isobutane. Alternative names for this group used in the petroleum industry are linear paraffins or n-paraffins.
The first eight members of the series (in terms of number of carbon atoms) are named as follows:
- methane
- CH4 – one carbon and 4 hydrogen
- ethane
- C2H6 – two carbon and 6 hydrogen
- propane
- C3H8 – three carbon and 8 hydrogen
- butane
- C4H10 – four carbon and 10 hydrogen
- pentane
- C5H12 – five carbon and 12 hydrogen
- hexane
- C6H14 – six carbon and 14 hydrogen
- heptane
- C7H16 – seven carbons and 16 hydrogen
- octane
- C8H18 – eight carbons and 18 hydrogen
The first four names were derived from methanol, ether, propionic acid and butyric acid. Alkanes with five or more carbon atoms are named by adding the suffix -ane to the appropriate numerical multiplier prefix[8] with elision of any terminal vowel (-a or -o) from the basic numerical term. Hence, pentane, C5H12; hexane, C6H14; heptane, C7H16; octane, C8H18; etc. The numeral prefix is generally Greek; however, alkanes with a carbon atom count ending in nine, for example nonane, use the Latin prefix non-.
Branched alkanes
Simple branched alkanes often have a common name using a prefix to distinguish them from linear alkanes, for example n-pentane, isopentane, and neopentane.
IUPAC naming conventions can be used to produce a systematic name.
The key steps in the naming of more complicated branched alkanes are as follows:[9]
- Identify the longest continuous chain of carbon atoms
- Name this longest root chain using standard naming rules
- Name each side chain by changing the suffix of the name of the alkane from "-ane" to "-yl"
- Number the longest continuous chain in order to give the lowest possible numbers for the side-chains[10]
- Number and name the side chains before the name of the root chain
- If there are multiple side chains of the same type, use prefixes such as "di-" and "tri-" to indicate it as such, and number each one.
- Add side chain names in alphabetical (disregarding "di-" etc. prefixes) order in front of the name of the root chain
| Common name | n-pentane | isopentane | neopentane |
|---|---|---|---|
| IUPAC name | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Structure |  |
|
Saturated cyclic hydrocarbons
Though technically distinct from the alkanes, this class of hydrocarbons is referred to by some as the "cyclic alkanes." As their description implies, they contain one or more rings.
Simple cycloalkanes have a prefix "cyclo-" to distinguish them from alkanes. Cycloalkanes are named as per their acyclic counterparts with respect to the number of carbon atoms in their backbones, e.g., cyclopentane (C5H10) is a cycloalkane with 5 carbon atoms just like pentane (C5H12), but they are joined up in a five-membered ring. In a similar manner, propane and cyclopropane, butane and cyclobutane, etc.
Substituted cycloalkanes are named similarly to substituted alkanes – the cycloalkane ring is stated, and the substituents are according to their position on the ring, with the numbering decided by the Cahn–Ingold–Prelog priority rules.[8]
Trivial/common names
The trivial (non-systematic) name for alkanes is 'paraffins'. Together, alkanes are known as the 'paraffin series'. Trivial names for compounds are usually historical artifacts. They were coined before the development of systematic names, and have been retained due to familiar usage in industry. Cycloalkanes are also called naphthenes.[11][12]
Branched-chain alkanes are called isoparaffins. "Paraffin" is a general term and often does not distinguish between pure compounds and mixtures of isomers, i.e., compounds of the same chemical formula, e.g., pentane and isopentane.
- In IUPAC
The following trivial names are retained in the IUPAC system:
- isobutane for 2-methylpropane
- isopentane for 2-methylbutane
- neopentane for 2,2-dimethylpropane.
- Non-IUPAC
Some non-IUPAC trivial names are occasionally used:
- cetane, for hexadecane
- cerane, for hexacosane[13]
Physical properties
All alkanes are colorless.[14][15] Alkanes with the lowest molecular weights are gases, those of intermediate molecular weight are liquids, and the heaviest are waxy solids.[16][17]
Table of alkanes
| Alkane | Formula | Boiling point[note 1] |
Melting point[note 1] |
Density[note 1] (at 20 °C) |
Isomers[note 2] |
|---|---|---|---|---|---|
| Methane | CH4 | −162 | −182 | 0.656 (gas) | 1 |
| Ethane | C2H6 | −89 | −183 | 1.26 (gas) | 1 |
| Propane | C3H8 | −42 | −188 | 2.01 (gas) | 1 |
| Butane | C4H10 | 0 | −138 | 2.48 (gas) | 2 |
| Pentane | C5H12 | 36 | −130 | 626 (liquid) | 3 |
| Hexane | C6H14 | 69 | −95 | 659 (liquid) | 5 |
| Heptane | C7H16 | 98 | −91 | 684 (liquid) | 9 |
| Octane | C8H18 | 126 | −57 | 703 (liquid) | 18 |
| Nonane | C9H20 | 151 | −54 | 718 (liquid) | 35 |
| Decane | C10H22 | 174 | −30 | 730 (liquid) | 75 |
| Undecane | C11H24 | 196 | −26 | 740 (liquid) | 159 |
| Dodecane | C12H26 | 216 | −10 | 749 (liquid) | 355 |
| Tridecane | C13H28 | 235 | −5.4 | 756 (liquid) | 802 |
| Tetradecane | C14H30 | 253 | 5.9 | 763 (liquid) | 1858 |
| Pentadecane | C15H32 | 270 | 10 | 769 (liquid) | 4347 |
| Hexadecane | C16H34 | 287 | 18 | 773 (liquid) | 10,359 |
| Heptadecane | C17H36 | 303 | 22 | 777 (solid) | 24,894 |
| Octadecane | C18H38 | 317 | 28 | 781 (solid) | 60,523 |
| Nonadecane | C19H40 | 330 | 32 | 785 (solid) | 148,284 |
| Icosane | C20H42 | 343 | 37 | 789 (solid) | 366,319 |
| Triacontane | C30H62 | 450 | 66 | 810 (solid) | 4,111,846,763 |
| Tetracontane | C40H82 | 525 | 82 | 817 (solid) | 62,481,801,147,341 |
| Pentacontane | C50H102 | 575 | Zdroj:https://en.wikipedia.org?pojem=Alkane


