
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Butanoic acid[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.003.212 | ||
| EC Number |
| ||
| |||
| KEGG |
| ||
| MeSH | Butyric+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 2820 | ||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| C 3H 7COOH | |||
| Molar mass | 88.106 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Unpleasant, similar to vomit or body odor | ||
| Density | 1.135 g/cm3 (−43 °C)[2] 0.9528 g/cm3 (25 °C)[3] | ||
| Melting point | −5.1 °C (22.8 °F; 268.0 K)[3] | ||
| Boiling point | 163.75 °C (326.75 °F; 436.90 K)[3] | ||
| Sublimes at −35 °C ΔsublH | |||
| Miscible | |||
| Solubility | Miscible with ethanol, ether. Slightly soluble in CCl4 | ||
| log P | 0.79 | ||
| Vapor pressure | 0.112 kPa (20 °C) 0.74 kPa (50 °C) 9.62 kPa (100 °C)[4] | ||
Henry's law
constant (kH) |
5.35·10−4 L·atm/mol | ||
| Acidity (pKa) | 4.82 | ||
| −55.10·10−6 cm3/mol | |||
| Thermal conductivity | 1.46·105 W/m·K | ||
Refractive index (nD)
|
1.398 (20 °C)[3] | ||
| Viscosity | 1.814 cP (15 °C)[5] 1.426 cP (25 °C) | ||
| Structure | |||
| Monoclinic (−43 °C)[2] | |||
| C2/m[2] | |||
a = 8.01 Å, b = 6.82 Å, c = 10.14 Å[2] α = 90°, β = 111.45°, γ = 90°
| |||
| 0.93 D (20 °C)[5] | |||
| Thermochemistry | |||
Heat capacity (C)
|
178.6 J/mol·K[4] | ||
Std molar
entropy (S⦵298) |
222.2 J/mol·K[5] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−533.9 kJ/mol[4] | ||
Std enthalpy of
combustion (ΔcH⦵298) |
2183.5 kJ/mol[4] | ||
| Hazards | |||
| GHS labelling: | |||
 [6] [6]
| |||
| Danger | |||
| H314[6] | |||
| P280, P305+P351+P338, P310[6] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 71 to 72 °C (160 to 162 °F; 344 to 345 K)[6] | ||
| 440 °C (824 °F; 713 K)[6] | |||
| Explosive limits | 2.2–13.4% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
2000 mg/kg (oral, rat) | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related carboxylic acids
|
Propionic acid, Pentanoic acid | ||
Related compounds
|
1-Butanol Butyraldehyde Methyl butyrate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
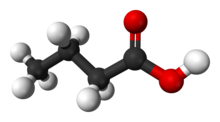
Butyric acid (/ˈbjuːtɪrɪk/; from Ancient Greek: βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH3CH2CH2CO2H. It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-methylpropanoic acid) is an isomer. Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a common industrial chemical[7] and an important component in the mammalian gut.
History
Butyric acid was first observed in an impure form in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. However, Chevreul did not publish his early research on butyric acid; instead, he deposited his findings in manuscript form with the secretary of the Academy of Sciences in Paris, France. Henri Braconnot, a French chemist, was also researching the composition of butter and was publishing his findings and this led to disputes about priority. As early as 1815, Chevreul claimed that he had found the substance responsible for the smell of butter.[8] By 1817, he published some of his findings regarding the properties of butyric acid and named it.[9] However, it was not until 1823 that he presented the properties of butyric acid in detail.[10] The name butyric acid comes from βούτῡρον, meaning "butter", the substance in which it was first found. The Latin name butyrum (or buturum) is similar.
Occurrence
Triglycerides of butyric acid compose 3–4% of butter. When butter goes rancid, butyric acid is liberated from the glyceride by hydrolysis.[11] It is one of the fatty acid subgroup called short-chain fatty acids. Butyric acid is a typical carboxylic acid that reacts with bases and affects many metals.[12] It is found in animal fat and plant oils, bovine milk, breast milk, butter, parmesan cheese, body odor, vomit and as a product of anaerobic fermentation (including in the colon).[13][14] It has a taste somewhat like butter and an unpleasant odor. Mammals with good scent detection abilities, such as dogs, can detect it at 10 parts per billion, whereas humans can detect it only in concentrations above 10 parts per million. In food manufacturing, it is used as a flavoring agent.[15]
In humans, butyric acid is one of two primary endogenous agonists of human hydroxycarboxylic acid receptor 2 (HCA2), a Gi/o-coupled G protein-coupled receptor.[16][17]
Butyric acid is present as its octyl ester in parsnip (Pastinaca sativa)[18] and in the seed of the ginkgo tree.[19]
Production
Industrial
In industry, butyric acid is produced by hydroformylation from propene and syngas, forming butyraldehyde, which is oxidised to the final product.[7]
- H2 + CO + CH3CH=CH2 → CH3CH2CH2CHObutyric acid
It can be separated from aqueous solutions by saturation with salts such as calcium chloride. The calcium salt, Ca(C4H7O2)2 · H2O, is less soluble in hot water than in cold.
Microbial biosynthesis

Butyrate is produced by several fermentation processes performed by obligate anaerobic bacteria.[20] This fermentation pathway was discovered by Louis Pasteur in 1861. Examples of butyrate-producing species of bacteria:
- Clostridium butyricum
- Clostridium kluyveri
- Clostridium pasteurianum
- Faecalibacterium prausnitzii
- Fusobacterium nucleatum
- Butyrivibrio fibrisolvens
- Eubacterium limosum
The pathway starts with the glycolytic cleavage of glucose to two molecules of pyruvate, as happens in most organisms. Pyruvate is oxidized into acetyl coenzyme A catalyzed by pyruvate:ferredoxin oxidoreductase. Two molecules of carbon dioxide (CO2) and two molecules of hydrogen (H2) are formed as waste products. Subsequently, ATP is produced in the last step of the fermentation. Three molecules of ATP are produced for each glucose molecule, a relatively high yield. The balanced equation for this fermentation is
- C6H12O6 → C4H8O2 + 2CO2 + 2H2
Other pathways to butyrate include succinate reduction and crotonate disproportionation.
| Action | Responsible enzyme |
|---|---|
| Acetyl coenzyme A converts into acetoacetyl coenzyme A | acetyl-CoA-acetyl transferase |
| Acetoacetyl coenzyme A converts into β-hydroxybutyryl CoA | β-hydroxybutyryl-CoA dehydrogenase |
| β-hydroxybutyryl CoA converts into crotonyl CoA | crotonase |
| Crotonyl CoA converts into butyryl CoA (CH3CH2CH2C=O−CoA) | butyryl CoA dehydrogenase |
| A phosphate group replaces CoA to form butyryl phosphate | phosphobutyrylase |
| The phosphate group joins ADP to form ATP and butyrate | butyrate kinase |
Several species form acetone and n-butanol in an alternative pathway, which starts as butyrate fermentation. Some of these species are:
- Clostridium acetobutylicum, the most prominent acetone and butanol producer, used also in industry
- Clostridium beijerinckii
- Clostridium tetanomorphum
- Clostridium aurantibutyricum
These bacteria begin with butyrate fermentation, as described above, but, when the pH drops below 5, they switch into butanol and acetone production to prevent further lowering of the pH. Two molecules of butanol are formed for each molecule of acetone.
The change in the pathway occurs after acetoacetyl CoA formation. This intermediate then takes two possible pathways:
- acetoacetyl CoA → acetoacetate → acetone
- acetoacetyl CoA → butyryl CoA → butyraldehyde → butanol
For commercial purposes Clostridium species are used preferably for butyric acid or butanol production. The most common species used for probiotics is the Clostridium butyricum.[21]
Fermentable fiber sources
Highly-fermentable fiber residues, such as those from resistant starch, oat bran, pectin, and guar are transformed by colonic bacteria into short-chain fatty acids (SCFA) including butyrate, producing more SCFA than less fermentable fibers such as celluloses.[14][22] One study found that resistant starch consistently produces more butyrate than other types of dietary fiber.[23] The production of SCFA from fibers in ruminant animals such as cattle is responsible for the butyrate content of milk and butter.[13][24]
Fructans are another source of prebiotic soluble dietary fibers which can be digested to produce butyrate.[25] They are often found in the soluble fibers of foods which are high in sulfur, such as the allium and cruciferous vegetables. Sources of fructans include wheat (although some wheat strains such as spelt contain lower amounts),[26] rye, barley, onion, garlic, Jerusalem and globe artichoke, asparagus, beetroot, chicory, dandelion leaves, leek, radicchio, the white part of spring onion, broccoli, brussels sprouts, cabbage, fennel, and prebiotics, such as fructooligosaccharides (FOS), oligofructose, and inulin.[27][28]
Reactions
Butyric acid reacts as a typical carboxylic acid: it can form amide, ester, anhydride, and chloride derivatives.[29] The latter, butyryl chloride, is commonly used as the intermediate to obtain the others.
Uses
Butyric acid is used in the preparation of various butyrate esters. It is used to produce cellulose acetate butyrate (CAB), which is used in a wide variety of tools, paints, and coatings, and is more resistant to degradation than cellulose acetate.[30] CAB can degrade with exposure to heat and moisture, releasing butyric acid.[31]
Low-molecular-weight esters of butyric acid, such as methyl butyrate, have mostly pleasant aromas or tastes.[7] As a consequence, they are used as food and perfume additives. It is an approved food flavoring in the EU FLAVIS database (number 08.005).
Due to its powerful odor, it has also been used as a fishing bait additive.[32] Many of the commercially available flavors used in carp (Cyprinus carpio) baits use butyric acid as their ester base. It is not clear whether fish are attracted by the butyric acid itself or the substances added to it. Butyric acid was one of the few organic acids shown to be palatable for both tench and bitterling.[33] The substance has been used as a stink bomb by the Sea Shepherd Conservation Society to disrupt Japanese whaling crews.[34]
Pharmacology
| Inhibited enzyme | IC50 (nM) | Entry note |
|---|---|---|
| HDAC1 | 16,000 | |
| HDAC2 | 12,000 | |
| HDAC3 | 9,000 | |
| HDAC4 | 2,000,000 | Lower bound |
| HDAC5 | 2,000,000 | Lower bound |
| HDAC6 | 2,000,000 | Lower bound |
| HDAC7 | 2,000,000 | Lower bound |
| HDAC8 | 15,000 | |
| HDAC9 | 2,000,000 | Lower bound |
| CA1 | 511,000 | |
| CA2 | 1,032,000 | |
| GPCR target | pEC50 | Entry note |
| FFAR2 | 2.9–4.6 | Full agonist |
| FFAR3 | 3.8–4.9 | Full agonist |
| HCA2 | 2.8 | Agonist |
Pharmacodynamics
Butyric acid (pKa 4.82) is fully ionized at physiological pH, so its anion is the material that is mainly relevant in biological systems. It is one of two primary endogenous agonists of human hydroxycarboxylic acid receptor 2 (HCA2, also known as GPR109A), a Gi/o-coupled G protein-coupled receptor (GPCR),[16][17]
Like other short-chain fatty acids (SCFAs), butyrate is an agonist at the free fatty acid receptors FFAR2 and FFAR3, which function as nutrient sensors that facilitate the homeostatic control of energy balance; however, among the group of SCFAs, only butyrate is an agonist of HCA2.[37][38][39] It is also an HDAC inhibitor (specifically, HDAC1, HDAC2, HDAC3, and HDAC8),[35][36] a drug that inhibits the function of histone deacetylase enzymes, thereby favoring an acetylated state of histones in cells.[39] Histone acetylation loosens the structure of chromatin by reducing the electrostatic attraction between histones and DNA.[39] In general, it is thought that transcription factors will be unable to access regions where histones are tightly associated with DNA (i.e., non-acetylated, e.g., heterochromatin).[medical citation needed] Therefore, butyric acid is thought to enhance the transcriptional activity at promoters,[39] which are typically silenced or downregulated due to histone deacetylase activity.
Pharmacokinetics
Butyrate that is produced in the colon through microbial fermentation of dietary fiber is primarily absorbed and metabolized by colonocytes and the liver[note 1] for the generation of ATP during energy metabolism; however, some butyrate is absorbed in the distal colon, which is not connected to the portal vein, thereby allowing for the systemic distribution of butyrate to multiple organ systems through the circulatory system.[39][40] Butyrate that has reached systemic circulation can readily cross the blood–brain barrier via monocarboxylate transporters (i.e., certain members of the SLC16A group of transporters).[41][42] Other transporters that mediate the passage of butyrate across lipid membranes include SLC5A8 (SMCT1), SLC27A1 (FATP1), and SLC27A4 (FATP4).[35][42]
Metabolism
Butyric acid is metabolized by various human XM-ligases (ACSM1, ACSM2B, ASCM3, ACSM4, ACSM5, and ACSM6), also known as butyrate–CoA ligase.[43][44] The metabolite produced by this reaction is butyryl–CoA, and is produced as follows:[43]
- Adenosine triphosphate + butyric acid + coenzyme A → adenosine monophosphate + pyrophosphate + butyryl-CoA
As a short-chain fatty acid, butyrate is metabolized by mitochondria as an energy (i.e., adenosine triphosphate or ATP) source through fatty acid metabolism.[39] In particular, it is an important energy source for cells lining the mammalian colon (colonocytes).[25] Without butyrates, colon cells undergo autophagy (i.e., self-digestion) and die.[45]
In humans, the butyrate precursor tributyrin, which is naturally present in butter, is metabolized by triacylglycerol lipase into dibutyrin and butyrate through the reaction:[46]
- Tributyrin + H2O → dibutyrin + butyric acid
Biochemistry
Butyrate has numerous effects on energy homeostasis and related diseases (diabetes and obesity), inflammation, and immune function (e.g., it has pronounced antimicrobial and anticarcinogenic effects) in humans. These effects occur through its metabolism by mitochondria to generate ATP during fatty acid metabolism or through one or more of its histone-modifying enzyme targets (i.e., the class I histone deacetylases) and G-protein coupled receptor targets (i.e., FFAR2, FFAR3, and HCA2).[37][47]
Zdroj:https://en.wikipedia.org?pojem=Butyric_acidText je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk



