
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
In chemistry, an open-chain compound (or open chain compound) or acyclic compound (Greek prefix α 'without' and κύκλος 'cycle') is a compound with a linear structure, rather than a cyclic one.[1] An open-chain compound having no side groups is called a straight-chain compound (also spelled as straight chain compound).[2][3] Many of the simple molecules of organic chemistry, such as the alkanes and alkenes, have both linear and ring isomers, that is, both acyclic and cyclic. For those with 4 or more carbons, the linear forms can have straight-chain or branched-chain isomers. The lowercase prefix n- denotes the straight-chain isomer; for example, n-butane is straight-chain butane, whereas i-butane is isobutane. Cycloalkanes are isomers of alkenes, not of alkanes, because the ring's closure involves a C-C bond. Having no rings (aromatic or otherwise), all open-chain compounds are aliphatic.
Typically in biochemistry, some isomers are more prevalent than others. For example, in living organisms, the open-chain isomer of glucose usually exists only transiently, in small amounts; D-glucose is the usual isomer; and L-glucose is rare.
Straight-chain molecules are often not literally straight, in the sense that their bond angles are often not 180°, but the name reflects that they are schematically straight. For example, the straight-chain alkanes are wavy or "puckered", as the models below show.
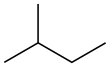
|
||
| branched-chain | straight-chain | cyclic |
| open-chain | ||


References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "polycyclic system". doi:10.1351/goldbook.P04724
- ^ Coles, Lydia (1968). "A chromatographic method for the separation of branched-chain and straight-chain compounds of columns containing urea". Journal of Chromatography A. 32 (4): 657–661. doi:10.1016/S0021-9673(01)80544-6. PMID 5645558.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "silazanes". doi:10.1351/goldbook.S05669
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk
