
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetraethylplumbane | |
| Other names
Lead tetraethyl
Tetraethyl lead | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | TEL |
| 3903146 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.979 |
| EC Number |
|
| 68951 | |
| MeSH | Tetraethyl+lead |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1649 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H20Pb | |
| Molar mass | 323.4 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | pleasant, sweet[1] |
| Density | 1.653 g cm−3 |
| Melting point | −136 °C (−213 °F; 137 K) |
| Boiling point | 84 to 85 °C (183 to 185 °F; 357 to 358 K) 15 mmHg |
| 200 parts per billion (ppb) (20 °C)[1] | |
| Vapor pressure | 0.2 mmHg (20 °C)[1] |
Refractive index (nD)
|
1.5198 |
| Structure | |
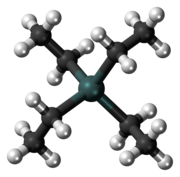
| Tetrahedral | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable, extremely toxic |
| GHS labelling: | |
  
| |
| H300+H310+H330, H360, H373, H410 | |
| P201, P202, P260, P262, P264, P270, P271, P273, P280, P281, P284, P301+P310, P302+P350, P304+P340, P308+P313, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 73 °C (163 °F; 346 K) |
| Explosive limits | 1.8%–?[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
35 mg/kg (rat, oral) 17 mg/kg (rat, oral) 12.3 mg/kg (rat, oral)[2] |
LDLo (lowest published)
|
30 mg/kg (rabbit, oral) 24 mg/kg (rat, oral)[2] |
LC50 (median concentration)
|
850 mg/m3 (rat, 1 hr)[2] |
LCLo (lowest published)
|
650 mg/m3 (mouse, 7 hr)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.075 mg/m3 [1] |
REL (Recommended)
|
TWA 0.075 mg/m3 [1] |
IDLH (Immediate danger)
|
40 mg/m3 (as Pb)[1] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula Pb(C2H5)4. It is a fuel additive, first being mixed with gasoline beginning in the 1920s as a patented octane rating booster that allowed engine compression to be raised substantially. This in turn increased vehicle performance and fuel economy.[3][4] TEL was first synthesised by German chemist Carl Jacob Löwig in 1853. American chemical engineer Thomas Midgley Jr., who was working for General Motors, was the first to discover its effectiveness as an antiknock agent in 1921, after spending several years attempting to find an additive that was both highly effective and inexpensive.
Concerns were later raised over the toxic effects of lead, especially on children.[5] On cars not designed to operate on leaded gasoline, lead and lead oxides coat the catalyst in catalytic converters, rendering them ineffective, and can sometimes foul spark plugs.[6] Starting in the 1970s, many countries began phasing out TEL in automotive fuel. In 2011 a study, backed by the United Nations, estimated that the removal of TEL had resulted in $2.4 trillion in annual benefits, and 1.2 million fewer premature deaths.[7]
TEL is still used as an additive in some grades of aviation fuel. Innospec has claimed to be the last firm legally making TEL but, as of 2013[update], TEL was being produced illegally by several companies in China.[8] In July 2021, the sale of leaded gasoline for cars was completely phased out worldwide, prompting the United Nations Environment Programme (UNEP) to declare an "official end" of its use in cars on August 30, 2021.[9]
Synthesis and properties
TEL is produced by reacting chloroethane with a sodium–lead alloy.[10][11]
The product is recovered by steam distillation, leaving a sludge of lead and sodium chloride.[12] TEL is a viscous colorless liquid with a sweet odor.[13] Because TEL is charge neutral and contains an exterior of alkyl groups, it is highly lipophilic and soluble in petrol (gasoline). This property, which allows it to dissolve so evenly and effectively in motor fuel, also allows it to dissolve oils and fats well, and therefore, diffuse through the blood–brain barrier and accumulate within the limbic forebrain, frontal cortex, and hippocampus.[14]
Despite decades of research, no reactions were found to improve upon this process, which is rather difficult, involves metallic sodium, and converts only 25% of the lead to TEL. A related compound, tetramethyllead, was commercially produced by a different electrolytic reaction.[10] A process with lithium was developed but never put into practice.[15]
Reactions
A noteworthy feature of TEL is the weakness of its four C–Pb bonds. At the temperatures found in internal combustion engines, TEL decomposes completely into lead as well as combustible, short-lived ethyl radicals. Lead and lead oxide scavenge radical intermediates in combustion reactions. Engine knock is caused by a cool flame, an oscillating low-temperature combustion reaction that occurs before the proper, hot ignition. Lead quenches the pyrolysed radicals and thus kills the radical chain reaction that would sustain a cool flame, preventing it from disturbing the smooth ignition of the hot flame front. Lead itself is the reactive antiknock agent, and the ethyl groups serve as a gasoline-soluble carrier.[10]
When TEL burns, it produces not only carbon dioxide and water, but also lead and lead(II) oxide:[16]
Pb and PbO would quickly over-accumulate and foul an engine. For this reason, 1,2-dichloroethane and 1,2-dibromoethane were also added to gasoline as lead scavengers—these agents form volatile lead(II) chloride and lead(II) bromide, respectively, which flush the lead from the engine and into the air:[16]
In motor fuel
TEL was extensively used as a gasoline additive beginning in the 1920s,[17] wherein it served as an effective antiknock agent and reduced exhaust valve and valve seat wear. Concerns were raised in reputable journals of likely health outcomes of fine particles of lead in the atmosphere.[18][19][20]
Valve wear preventive
Tetraethyllead helps cool intake valves and is an excellent buffer against microwelds forming between exhaust valves and their seats.[21] Once these valves reopen, the microwelds pull apart and abrade the valves and seats, leading to valve recession. When TEL began to be phased out, the automotive industry began specifying hardened valve seats and upgraded materials which allow for high wear resistance without requiring lead.[22]
Antiknock agent
A gasoline-fuelled reciprocating engine requires fuel of sufficient octane rating to prevent uncontrolled combustion (preignition and detonation).[10] Antiknock agents allow the use of higher compression ratios for greater efficiency[23] and peak power.[24] Adding varying amounts of additives to gasoline allowed easy, inexpensive control of octane ratings. TEL offered the business advantage of being commercially profitable because its use for this purpose could be patented.[17] Aviation fuels with TEL used in WWII reached octane ratings of 150 to enable turbocharged and supercharged engines such as the Rolls-Royce Merlin and Griffon to reach high horsepower ratings at altitude.[25] In military aviation, TEL manipulation allowed a range of different fuels to be tailored for particular flight conditions.[citation needed]
In 1935 a licence to produce TEL was given to IG Farben, enabling the newly formed German Luftwaffe to use high-octane gasoline. A company, Ethyl GmbH, was formed that produced TEL at two sites in Germany with a government contract from 10 June 1936.[26]
In 1938 the United Kingdom Air Ministry contracted with ICI for the construction and operation of a TEL plant. A site was chosen at Holford Moss, near Plumley in Cheshire. Construction started in April 1939 and TEL was being produced by September 1940.[27]
"Ethyl Fluid"

For mixing with raw gasoline, TEL was most commonly supplied in the form of "Ethyl Fluid", which consisted of TEL blended with 1,2-dichloroethane and 1,2-dibromoethane. Ethyl Fluid also contained a reddish dye to distinguish treated from untreated gasoline and discourage the use of leaded gasoline for other purposes such as cleaning.[28]
In the 1920s before safety procedures were strengthened, 17 workers for the Ethyl Corporation, DuPont, and Standard Oil died from the effects of exposure to lead.[17]
Ethyl Fluid's formulation consisted of:[10]
- 61.45% tetraethyllead
- 18.80% 1,2-dichloroethane
- 17.85% 1,2-dibromoethane
- 1.90% inerts and dyes
Dichloroethane and dibromoethane act in a synergistic manner, where equal or approximately equal quantities of both provide the best scavenging ability.[10]
Phaseout and ban
In most industrialized countries, a phaseout of TEL from road vehicle fuels was completed by the early 2000s because of concerns over air and soil lead levels and the accumulative neurotoxicity of lead. In the European Union, tetraethyllead has been classified as a Substance of Very High Concern and placed on the Candidate List for Authorisation under Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).[29] Potential use of TEL would need to be authorised through the REACH authorisation procedure. While not a complete ban, it introduces significant obligations such as a mandatory analysis of alternatives and socioeconomic analysis.[citation needed]
The use of catalytic converters, mandated in the United States for 1975 and later model-year cars to meet tighter emissions regulations, started a gradual phase-out of leaded gasoline in the U.S.[30] The need for TEL was lessened by several advances in automotive engineering and petroleum chemistry. Safer methods for making higher-octane blending stocks such as reformate and iso-octane reduced the need to rely on TEL, as did other antiknock additives of varying toxicity including metallic compounds such as methylcyclopentadienyl manganese tricarbonyl (MMT) as well as oxygenates including methyl tertiary-butyl ether (MTBE), tert-amyl methyl ether (TAME), and ethyl tert-butyl ether (ETBE).[citation needed]
The first country to completely ban leaded gasoline was Japan in 1986.[31]
Since January 1993 all gasoline powered cars sold in the European Union and the United Kingdom have been required to use unleaded fuel. This was to comply with the Euro 1 emission standards which mandated that all new cars to be fitted with a catalytic converter.[32] Unleaded fuel was first introduced in the United Kingdom in June 1986.[33]
Leaded gasoline was removed from the forecourts in the United Kingdom on January 1, 2000, and a Lead Replacement Petrol was introduced although this was largely withdrawn by 2003 due to dwindling sales.[34][35] An exemption to the ban exists for owners of classic cars.[citation needed]
Vehicles designed and built to run on leaded fuel often require modification to run on unleaded gasoline. These modifications fall into two categories: those required for physical compatibility with unleaded fuel, and those performed to compensate for the relatively low octane of early unleaded fuels. Physical compatibility requires the installation of hardened exhaust valves and seats. Compatibility with reduced octane was addressed by reducing compression, generally by installing thicker cylinder head gaskets and/or rebuilding the engine with compression-reducing pistons, although modern high-octane unleaded gasoline has eliminated the need to decrease compression ratios.[citation needed]
Leaded gasoline remained legal as of late 2014[36] in parts of Algeria, Iraq, Yemen, Myanmar, North Korea, and Afghanistan.[37][38][needs update] North Korea and Myanmar purchased their TEL from China, while Algeria, Iraq, and Yemen purchased it from the specialty chemical company Innospec, the world's sole remaining legal manufacturer of TEL.[39] In 2011 several Innospec executives were charged and imprisoned for bribing various government state-owned oil companies to approve the sale of their TEL products.[38][40]
As of June 2016[update] the UNEP-sponsored phase-out was nearly complete: only Algeria, Iraq, and Yemen continued widespread use of leaded gasoline, although not exclusively.[41] In July 2021, Algeria had halted its sale.[9]
Leaded-fuel bans
This section needs additional citations for verification. (May 2021) |
Leaded-fuel bans for road vehicles came into effect as follows:
Europe
|
North America
|
South America |
Asia
|
Oceania
|
Africa
- Egypt: 1999
- South Africa: 2006
- Leaded petrol was supposed to be completely phased out continent-wide on 1 January 2006, following a ban initiated from the 2002 Earth Summit.[78] However, in Algeria refineries needed to be altered; as a result, leaded fuel remained available in parts of Algeria,[38] with phaseout scheduled for 2016. After the Algerian Government outlawed the sale of leaded petrol throughout all of Algeria, leaded petrol has now been effectively phased out.[79][80]
In motor racing
Leaded fuel was commonly used in professional motor racing, until its phase out beginning in the 1990s. Since 1993, Formula One racing cars have been required to use fuel containing no more than 5 mg/L of lead.[81][verification needed]
NASCAR began experimentation in 1998 with an unleaded fuel, and in 2006 began switching the national series to unleaded fuel, completing the transition at the Fontana round in February 2007 when the premier class switched. This was influenced after blood tests of NASCAR teams revealed elevated blood lead levels.[82][83]
Aviation gasoline
TEL remains an ingredient of 100 octane avgas for piston-engine aircraft. The current formulation of 100LL (low lead, blue) aviation gasoline contains 2.12 grams per US gallon (0.56 g/L) of TEL, half the amount of the previous 100/130 (green) octane avgas (at 4.24 grams per gallon),[84] and twice as much as the 1 gram per gallon permitted in regular automotive leaded gasoline prior to 1988 and substantially greater than the allowed 0.001 grams per gallon in automotive unleaded gasoline sold in the United States today.[85] The United States Environmental Protection Agency, FAA, and others are working on economically feasible replacements for leaded avgas, which still releases 100 tons of lead every year.[86]
Alternative antiknock agents
Antiknock agents are classed as high-percentage additives, such as alcohol, and low-percentage additives based on heavy elements. Since the main problem with TEL is its lead content, many alternative additives that contain less poisonous metals have been examined. A manganese-carrying additive, methylcyclopentadienyl manganese tricarbonyl (MMT or methylcymantrene), was used for a time as an antiknock agent, though its safety is controversial and it has been the subject of bans and lawsuits. Ferrocene, an organometallic compound of iron, is also used as an antiknock agent although with some significant drawbacks.[87]
High-percentage additives are organic compounds that do not contain metals, but require much higher blending ratios, such as 20–30% for benzene and ethanol. It had been established by 1921 that ethanol was an effective antiknock agent, but TEL was introduced instead mainly for commercial reasons.[30] Oxygenates such as TAME derived from natural gas, MTBE made from methanol, and ethanol-derived ETBE, have largely supplanted TEL. MTBE has environmental risks of its own and there are also bans on its use.[citation needed]
Zdroj:https://en.wikipedia.org?pojem=Tetraethyllead
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk

