
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| Composition | Elementary particle |
|---|---|
| Statistics | Bosonic |
| Family | Gauge boson |
| Interactions | Electromagnetic, weak (and gravity) |
| Symbol | γ |
| Theorized | Albert Einstein (1905) The name "photon" is generally attributed to Gilbert N. Lewis (1926) |
| Mass | 0 (theoretical value) < 1×10−18 eV/c2 (experimental limit)[1] |
| Mean lifetime | Stable[1] |
| Electric charge | 0
< 1×10−35 e[1] |
| Color charge | 0 |
| Spin | 1 ħ |
| Spin states | +1 ħ, −1 ħ |
| Parity | −1[1] |
| C parity | −1[1] |
| Condensed | I(JP C)=0,1(1−−)[1] |
A photon (from Ancient Greek φῶς, φωτός (phôs, phōtós) 'light') is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that always move at the speed of light when in vacuum. The photon belongs to the class of boson particles.
As with other elementary particles, photons are best explained by quantum mechanics and exhibit wave–particle duality, their behavior featuring properties of both waves and particles.[2] The modern photon concept originated during the first two decades of the 20th century with the work of Albert Einstein, who built upon the research of Max Planck. While Planck was trying to explain how matter and electromagnetic radiation could be in thermal equilibrium with one another, he proposed that the energy stored within a material object should be regarded as composed of an integer number of discrete, equal-sized parts. To explain the photoelectric effect, Einstein introduced the idea that light itself is made of discrete units of energy. In 1926, Gilbert N. Lewis popularized the term photon for these energy units.[3][4][5] Subsequently, many other experiments validated Einstein's approach.[6][7][8]
In the Standard Model of particle physics, photons and other elementary particles are described as a necessary consequence of physical laws having a certain symmetry at every point in spacetime. The intrinsic properties of particles, such as charge, mass, and spin, are determined by gauge symmetry. The photon concept has led to momentous advances in experimental and theoretical physics, including lasers, Bose–Einstein condensation, quantum field theory, and the probabilistic interpretation of quantum mechanics. It has been applied to photochemistry, high-resolution microscopy, and measurements of molecular distances. Moreover, photons have been studied as elements of quantum computers, and for applications in optical imaging and optical communication such as quantum cryptography.
Nomenclature
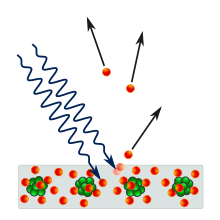

The word quanta (singular quantum, Latin for how much) was used before 1900 to mean particles or amounts of different quantities, including electricity. In 1900, the German physicist Max Planck was studying black-body radiation, and he suggested that the experimental observations, specifically at shorter wavelengths, would be explained if the energy stored within a molecule was a "discrete quantity composed of an integral number of finite equal parts", which he called "energy elements".[9] In 1905, Albert Einstein published a paper in which he proposed that many light-related phenomena—including black-body radiation and the photoelectric effect—would be better explained by modelling electromagnetic waves as consisting of spatially localized, discrete wave-packets.[10] He called such a wave-packet a light quantum (German: ein Lichtquant).[a]
The name photon derives from the Greek word for light, φῶς (transliterated phôs). Arthur Compton used photon in 1928, referring to Gilbert N. Lewis, who coined the term in a letter to Nature on 18 December 1926.[3][11] The same name was used earlier but was never widely adopted before Lewis: in 1916 by the American physicist and psychologist Leonard T. Troland, in 1921 by the Irish physicist John Joly, in 1924 by the French physiologist René Wurmser (1890–1993), and in 1926 by the French physicist Frithiof Wolfers (1891–1971).[5] The name was suggested initially as a unit related to the illumination of the eye and the resulting sensation of light and was used later in a physiological context. Although Wolfers's and Lewis's theories were contradicted by many experiments and never accepted, the new name was adopted by most physicists very soon after Compton used it.[5][b]
In physics, a photon is usually denoted by the symbol γ (the Greek letter gamma). This symbol for the photon probably derives from gamma rays, which were discovered in 1900 by Paul Villard,[13][14] named by Ernest Rutherford in 1903, and shown to be a form of electromagnetic radiation in 1914 by Rutherford and Edward Andrade.[15] In chemistry and optical engineering, photons are usually symbolized by hν, which is the photon energy, where h is the Planck constant and the Greek letter ν (nu) is the photon's frequency.[16]
Physical properties
The photon has no electric charge,[17][18] is generally considered to have zero rest mass[19] and is a stable particle. The experimental upper limit on the photon mass[20][21] is very small, on the order of 10−50 kg; its lifetime would be more than 1018 years.[22] For comparison the age of the universe is about 1.38×1010 years.
In a vacuum, a photon has two possible polarization states.[23] The photon is the gauge boson for electromagnetism,[24]: 29–30 and therefore all other quantum numbers of the photon (such as lepton number, baryon number, and flavour quantum numbers) are zero.[25] Also, the photon obeys Bose–Einstein statistics, and not Fermi–Dirac statistics. That is, they do not obey the Pauli exclusion principle[26]: 1221 and more than one can occupy the same bound quantum state.
Photons are emitted in many natural processes. For example, when a charge is accelerated it emits synchrotron radiation. During a molecular, atomic or nuclear transition to a lower energy level, photons of various energy will be emitted, ranging from radio waves to gamma rays. Photons can also be emitted when a particle and its corresponding antiparticle are annihilated (for example, electron–positron annihilation).[26]: 572, 1114, 1172
Relativistic energy and momentum
In empty space, the photon moves at c (the speed of light) and its energy and momentum are related by E = pc, where p is the magnitude of the momentum vector p. This derives from the following relativistic relation, with m = 0:[27]
The energy and momentum of a photon depend only on its frequency () or inversely, its wavelength (λ):
where k is the wave vector, where
- k ≡ |k| = 2π /λ is the wave number, and
- ω ≡ 2 πν is the angular frequency, and
- ħ ≡ h/ 2π is the reduced Planck constant.[28]
Since points in the direction of the photon's propagation, the magnitude of its momentum is
Polarization and spin angular momentum
The photon also carries spin angular momentum, which is related to photon polarization. (Beams of light also exhibit properties described as orbital angular momentum of light).
The angular momentum of the photon has two possible values, either +ħ or −ħ. These two possible values correspond to the two possible pure states of circular polarization. Collections of photons in a light beam may have mixtures of these two values; a linearly polarized light beam will act as if it were composed of equal numbers of the two possible angular momenta.[29]: 325
The spin angular momentum of light does not depend on its frequency, and was experimentally verified by C. V. Raman and S. Bhagavantam in 1931.[30]
Antiparticle annihilation
The collision of a particle with its antiparticle can create photons. In free space at least two photons must be created since, in the center of momentum frame, the colliding antiparticles have no net momentum, whereas a single photon always has momentum (determined by the photon's frequency or wavelength, which cannot be zero). Hence, conservation of momentum (or equivalently, translational invariance) requires that at least two photons are created, with zero net momentum.[c][31]: 64–65 The energy of the two photons, or, equivalently, their frequency, may be determined from conservation of four-momentum.
Seen another way, the photon can be considered as its own antiparticle (thus an "antiphoton" is simply a normal photon with opposite momentum, equal polarization, and 180° out of phase). The reverse process, pair production, is the dominant mechanism by which high-energy photons such as gamma rays lose energy while passing through matter.[32] That process is the reverse of "annihilation to one photon" allowed in the electric field of an atomic nucleus.
The classical formulae for the energy and momentum of electromagnetic radiation can be re-expressed in terms of photon events. For example, the pressure of electromagnetic radiation on an object derives from the transfer of photon momentum per unit time and unit area to that object, since pressure is force per unit area and force is the change in momentum per unit time.[33]
Experimental checks on photon mass
Current commonly accepted physical theories imply or assume the photon to be strictly massless. If photons were not purely massless, their speeds would vary with frequency, with lower-energy (redder) photons moving slightly slower than higher-energy photons. Relativity would be unaffected by this; the so-called speed of light, c, would then not be the actual speed at which light moves, but a constant of nature which is the upper bound on speed that any object could theoretically attain in spacetime.[34] Thus, it would still be the speed of spacetime ripples (gravitational waves and gravitons), but it would not be the speed of photons.
If a photon did have non-zero mass, there would be other effects as well. Coulomb's law would be modified and the electromagnetic field would have an extra physical degree of freedom. These effects yield more sensitive experimental probes of the photon mass than the frequency dependence of the speed of light. If Coulomb's law is not exactly valid, then that would allow the presence of an electric field to exist within a hollow conductor when it is subjected to an external electric field. This provides a means for precision tests of Coulomb's law.[35] A null result of such an experiment has set a limit of m ≲ 10−14 eV/c2.[36]
Sharper upper limits on the mass of light have been obtained in experiments designed to detect effects caused by the galactic vector potential. Although the galactic vector potential is large because the galactic magnetic field exists on great length scales, only the magnetic field would be observable if the photon is massless. In the case that the photon has mass, the mass term 1/2m2AμAμ would affect the galactic plasma. The fact that no such effects are seen implies an upper bound on the photon mass of m < 3×10−27 eV/c2.[37] The galactic vector potential can also be probed directly by measuring the torque exerted on a magnetized ring.[38] Such methods were used to obtain the sharper upper limit of 1.07×10−27 eV/c2 (the equivalent of 10−36 daltons) given by the Particle Data Group.[39]
These sharp limits from the non-observation of the effects caused by the galactic vector potential have been shown to be model-dependent.[40] If the photon mass is generated via the Higgs mechanism then the upper limit of m ≲ 10−14 eV/c2 from the test of Coulomb's law is valid.
Historical development

In most theories up to the eighteenth century, light was pictured as being made of particles. Since particle models cannot easily account for the refraction, diffraction and birefringence of light, wave theories of light were proposed by René Descartes (1637),[41] Robert Hooke (1665),[42] and Christiaan Huygens (1678);[43] however, particle models remained dominant, chiefly due to the influence of Isaac Newton.[44] In the early 19th century, Thomas Young and August Fresnel clearly demonstrated the interference and diffraction of light, and by 1850 wave models were generally accepted.[45] James Clerk Maxwell's 1865 prediction[46] that light was an electromagnetic wave – which was confirmed experimentally in 1888 by Heinrich Hertz's detection of radio waves[47] – seemed to be the final blow to particle models of light.

The Maxwell wave theory, however, does not account for all properties of light. The Maxwell theory predicts that the energy of a light wave depends only on its intensity, not on its frequency; nevertheless, several independent types of experiments show that the energy imparted by light to atoms depends only on the light's frequency, not on its intensity. For example, some chemical reactions are provoked only by light of frequency higher than a certain threshold; light of frequency lower than the threshold, no matter how intense, does not initiate the reaction. Similarly, electrons can be ejected from a metal plate by shining light of sufficiently high frequency on it (the photoelectric effect); the energy of the ejected electron is related only to the light's frequency, not to its intensity.[48][d]
At the same time, investigations of black-body radiation carried out over four decades (1860–1900) by various researchers[50] culminated in Max Planck's hypothesis[51][52] that the energy of any system that absorbs or emits electromagnetic radiation of frequency ν is an integer multiple of an energy quantum E = hν . As shown by Albert Einstein,[10][53] some form of energy quantization must be assumed to account for the thermal equilibrium observed between matter and electromagnetic radiation; for this explanation of the photoelectric effect, Einstein received the 1921 Nobel Prize in physics.[54]
Since the Maxwell theory of light allows for all possible energies of electromagnetic radiation, most physicists assumed initially that the energy quantization resulted from some unknown constraint on the matter that absorbs or emits the radiation. In 1905, Einstein was the first to propose that energy quantization was a property of electromagnetic radiation itself.[10] Although he accepted the validity of Maxwell's theory, Einstein pointed out that many anomalous experiments could be explained if the energy of a Maxwellian light wave were localized into point-like quanta that move independently of one another, even if the wave itself is spread continuously over space.[10] In 1909[53] and 1916,[55] Einstein showed that, if Planck's law regarding black-body radiation is accepted, the energy quanta must also carry momentum p = h / λ , making them full-fledged particles. This photon momentum was observed experimentally by Arthur Compton,[56] for which he received the Nobel Prize in 1927. The pivotal question then, was how to unify Maxwell's wave theory of light with its experimentally observed particle nature. The answer to this question occupied Albert Einstein for the rest of his life,[57] and was solved in quantum electrodynamics and its successor, the Standard Model. (See § Quantum field theory and § As a gauge boson, below.)

Einstein's 1905 predictions were verified experimentally in several ways in the first two decades of the 20th century, as recounted in Robert Millikan's Nobel lecture.[58] However, before Compton's experiment[56] showed that photons carried momentum proportional to their wave number (1922),[full citation needed] most physicists were reluctant to believe that electromagnetic radiation itself might be particulate. (See, for example, the Nobel lectures of Wien,[50] Planck[52] and Millikan.)[58] Instead, there was a widespread belief that energy quantization resulted from some unknown constraint on the matter that absorbed or emitted radiation. Attitudes changed over time. In part, the change can be traced to experiments such as those revealing Compton scattering, where it was much more difficult not to ascribe quantization to light itself to explain the observed results.[59]
Even after Compton's experiment, Niels Bohr, Hendrik Kramers and John Slater made one last attempt to preserve the Maxwellian continuous electromagnetic field model of light, the so-called BKS theory.[60] An important feature of the BKS theory is how it treated the conservation of energy and the conservation of momentum. In the BKS theory, energy and momentum are only conserved on the average across many interactions between matter and radiation. However, refined Compton experiments showed that the conservation laws hold for individual interactions.[61] Accordingly, Bohr and his co-workers gave their model "as honorable a funeral as possible".[57] Nevertheless, the failures of the BKS model inspired Werner Heisenberg in his development of matrix mechanics.[62]
A few physicists persisted[63] in developing semiclassical models in which electromagnetic radiation is not quantized, but matter appears to obey the laws of quantum mechanics. Although the evidence from chemical and physical experiments for the existence of photons was overwhelming by the 1970s, this evidence could not be considered as absolutely definitive; since it relied on the interaction of light with matter, and a sufficiently complete theory of matter could in principle account for the evidence. Nevertheless, all semiclassical theories were refuted definitively in the 1970s and 1980s by photon-correlation experiments.[e] Hence, Einstein's hypothesis that quantization is a property of light itself is considered to be proven.
Wave–particle duality and uncertainty principles

Photons obey the laws of quantum mechanics, and so their behavior has both wave-like and particle-like aspects. When a photon is detected by a measuring instrument, it is registered as a single, particulate unit. However, the probability of detecting a photon is calculated by equations that describe waves. This combination of aspects is known as wave–particle duality. For example, the probability distribution for the location at which a photon might be detected displays clearly wave-like phenomena such as diffraction and interference. A single photon passing through a double slit has its energy received at a point on the screen with a probability distribution given by its interference pattern determined by Maxwell's wave equations.[66] However, experiments confirm that the photon is not a short pulse of electromagnetic radiation; a photon's Maxwell waves will diffract, but photon energy does not spread out as it propagates, nor does this energy divide when it encounters a beam splitter.[67] Rather, the received photon acts like a point-like particle since it is absorbed or emitted as a whole by arbitrarily small systems, including systems much smaller than its wavelength, such as an atomic nucleus (≈10−15 m across) or even the point-like electron.
While many introductory texts treat photons using the mathematical techniques of non-relativistic quantum mechanics, this is in some ways an awkward oversimplification, as photons are by nature intrinsically relativistic. Because photons have zero rest mass, no wave function defined for a photon can have all the properties familiar from wave functions in non-relativistic quantum mechanics.[f] In order to avoid these difficulties, physicists employ the second-quantized theory of photons described below, quantum electrodynamics, in which photons are quantized excitations of electromagnetic modes.[72]
Another difficulty is finding the proper analogue for the uncertainty principle, an idea frequently attributed to Heisenberg, who introduced the concept in analyzing a thought experiment involving an electron and a high-energy photon. However, Heisenberg did not give precise mathematical definitions of what the "uncertainty" in these measurements meant. The precise mathematical statement of the position–momentum uncertainty principle is due to Kennard, Pauli, and Weyl.[73][74] The uncertainty principle applies to situations where an experimenter has a choice of measuring either one of two "canonically conjugate" quantities, like the position and the momentum of a particle. According to the uncertainty principle, no matter how the particle is prepared, it is not possible to make a precise prediction for both of the two alternative measurements: if the outcome of the position measurement is made more certain, the outcome of the momentum measurement becomes less so, and vice versa.[75] A coherent state minimizes the overall uncertainty as far as quantum mechanics allows.[72] Quantum optics makes use of coherent states for modes of the electromagnetic field. There is a tradeoff, reminiscent of the position–momentum uncertainty relation, between measurements of an electromagnetic wave's amplitude and its phase.[72] This is sometimes informally expressed in terms of the uncertainty in the number of photons present in the electromagnetic wave, , and the uncertainty in the phase of the wave, . However, this cannot be an uncertainty relation of the Kennard–Pauli–Weyl type, since unlike position and momentum, the phase cannot be represented by a Hermitian operator.[76]
Bose–Einstein model of a photon gas
In 1924, Satyendra Nath Bose derived Planck's law of black-body radiation without using any electromagnetism, but rather by using a modification of coarse-grained counting of phase space.[77] Einstein showed that this modification is equivalent to assuming that photons are rigorously identical and that it implied a "mysterious non-local interaction",[78][79] now understood as the requirement for a symmetric quantum mechanical state. This work led to the concept of coherent states and the development of the laser. In the same papers, Einstein extended Bose's formalism to material particles (bosons) and predicted that they would condense into their lowest quantum state at low enough temperatures; this Bose–Einstein condensation was observed experimentally in 1995.[80] It was later used by Lene Hau to slow, and then completely stop, light in 1999[81] and 2001.[82]
The modern view on this is that photons are, by virtue of their integer spin, bosons (as opposed to fermions with half-integer spin). By the spin-statistics theorem, all bosons obey Bose–Einstein statistics (whereas all fermions obey Fermi–Dirac statistics).[83]
Stimulated and spontaneous emission

In 1916, Albert Einstein showed that Planck's radiation law could be derived from a semi-classical, statistical treatment of photons and atoms, which implies a link between the rates at which atoms emit and absorb photons. The condition follows from the assumption that functions of the emission and absorption of radiation by the atoms are independent of each other, and that thermal equilibrium is made by way of the radiation's interaction with the atoms. Consider a cavity in thermal equilibrium with all parts of itself and filled with electromagnetic radiation and that the atoms can emit and absorb that radiation. Thermal equilibrium requires that the energy density of photons with frequency (which is proportional to their number density) is, on average, constant in time; hence, the rate at which photons of any particular frequency are emitted must equal the rate at which they are absorbed.[84]
Einstein began by postulating simple proportionality relations for the different reaction rates involved. In his model, the rate for a system to absorb a photon of frequency and transition from a lower energy to a higher energy is proportional to the number of atoms with energy and to the energy density of ambient photons of that frequency,
where is the rate constant for absorption. For the reverse process, there are two possibilities: spontaneous emission of a photon, or the emission of a photon initiated by the interaction of the atom with a passing photon and the return of the atom to the lower-energy state. Following Einstein's approach, the corresponding rate for the emission of photons of frequency and transition from a higher energy to a lower energy is
where is the rate constant for emitting a photon spontaneously, and is the rate constant for emissions in response to ambient photons (induced or stimulated emission). In thermodynamic equilibrium, the number of atoms in state and those in state must, on average, be constant; hence, the rates and must be equal. Also, by arguments analogous to the derivation of Boltzmann statistics, the ratio of and is
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk























