
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
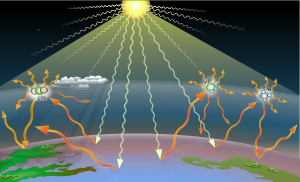

Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect.[1] The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F),[2] rather than the present average of 15 °C (59 °F).[3][4]
The five most abundant greenhouse gases in Earth's atmosphere, listed in decreasing order of average global mole fraction, are:[5][6] water vapor, carbon dioxide, methane, nitrous oxide, ozone. Other greenhouse gases of concern include chlorofluorocarbons (CFCs and HCFCs), hydrofluorocarbons (HFCs), perfluorocarbons, SF
6, and NF
3. Water vapor causes about half of the greenhouse effect, but humans are not directly adding to its amount,[7] so it is not a driver of climate change.[8]
Carbon dioxide is causing about three-quarters of global warming and can take thousands of years to be fully absorbed by the carbon cycle.[9][10] Methane causes most of the remaining warming and lasts in the atmosphere for an average of 12 years.[11] Human activities since the beginning of the Industrial Revolution (around 1750) have increased carbon dioxide by over 50%,[12] up to a level not seen in over 3 million years.[13] The atmospheric methane concentrations have increased by over 150% during the same time period.[14]
Without human influence, the natural flows of carbon between the atmosphere, terrestrial ecosystems, the ocean, and sediments would be fairly balanced.[15][16] The vast majority of carbon dioxide emissions by humans come from the burning of fossil fuels. Further contributions come from agriculture and industry.[17]: 687 [18][19] Methane emissions originate from agriculture, fossil fuel production, waste, and other sources.[20] If current emission rates continue then global warming will surpass 2.0 °C (3.6 °F) sometime between 2040 and 2070. This is a level which the Intergovernmental Panel on Climate Change (IPCC) says is "dangerous".[21]
Properties and mechanisms

Greenhouse gases are infrared active, meaning that they absorb and emit infrared radiation in the same long wavelength range as what is emitted by the Earth's surface, clouds and atmosphere.[22]: 2233
99% of the Earth's dry atmosphere (excluding water vapor) is made up of nitrogen (N
2) (78%) and oxygen (O
2) (21%). Because their molecules contain two atoms of the same element, they have no asymmetry in the distribution of their electrical charges,[23] and so are almost totally unaffected by infrared thermal radiation,[24] with only an extremely minor effect from collision-induced absorption.[25][26][27] A further 0.9% of the atmosphere is made up by argon (Ar), which is monatomic, and so completely transparent to thermal radiation. On the other hand, carbon dioxide (0.04%), methane, nitrous oxide and even less abundant trace gases account for less than 0.1% of Earth's atmosphere, but because their molecules contain atoms of different elements, there is an asymmetry in electric charge distribution which allows molecular vibrations to interact with electromagnetic radiation. This makes them infrared active, and so their presence causes greenhouse effect.[23]
Radiative forcing

Earth absorbs some of the radiant energy received from the sun, reflects some of it as light and reflects or radiates the rest back to space as heat. A planet's surface temperature depends on this balance between incoming and outgoing energy. When Earth's energy balance is shifted, its surface becomes warmer or cooler, leading to a variety of changes in global climate.[28] Radiative forcing is a metric calculated in watts per square meter, which characterizes the impact of an external change in a factor that influences climate. It is calculated as the difference in top-of-atmosphere (TOA) energy balance immediately caused by such an external change A positive forcing, such as from increased concentrations of greenhouse gases, means more energy arriving than leaving at the top-of-atmosphere, which causes additional warming, while negative forcing, like from sulfates forming in the atmosphere from sulfur dioxide, leads to cooling.[22]: 2245 [29]
Within the lower atmosphere, greenhouse gases exchange thermal radiation with the surface and limit radiative heat flow away from it, which reduces the overall rate of upward radiative heat transfer.[30]: 139 [31] The increased concentration of greenhouse gases is also cooling the upper atmosphere, as it is much thinner than the lower layers, and any heat re-emitted from greenhouse gases is more likely to travel further to space than to interact with the fewer gas molecules in the upper layers. The upper atmosphere is also shrinking as the result.[32]
Contributions of specific gases to the greenhouse effect
Anthropogenic changes to the natural greenhouse effect are sometimes referred to as the enhanced greenhouse effect.[22]: 2223
This table shows the most important contributions to the overall greenhouse effect, without which the average temperature of Earth's surface would be about −18 °C (0 °F),[2] instead of around 15 °C (59 °F).[3] This table also specifies tropospheric ozone, because this gas has a cooling effect in the stratosphere, but a warming influence comparable to nitrous oxide and CFCs in the troposphere.[33]
| K&T (1997)[34] | Schmidt (2010)[35] | |||
|---|---|---|---|---|
| Contributor | Clear Sky | With Clouds | Clear Sky | With Clouds |
| Water vapor | 60 | 41 | 67 | 50 |
| Clouds | 31 | 25 | ||
| CO2 | 26 | 18 | 24 | 19 |
| Tropospheric ozone (O3) | 8 | |||
| N2O + CH4 | 6 | |||
| Other | 9 | 9 | 7 | |
|
K&T (1997) used 353 ppm CO2 and calculated 125 W/m2 total clear-sky greenhouse effect; relied on single atmospheric profile and cloud model. "With Clouds" percentages are from Schmidt (2010) interpretation of K&T (1997). | ||||
Special role of water vapor

Water vapor is the most important greenhouse gas overall, being responsible for 41–67% of the greenhouse effect,[34][35] but its global concentrations are not directly affected by human activity. While local water vapor concentrations can be affected by developments such as irrigation, it has little impact on the global scale due to its short residence time of about nine days.[37] Indirectly, an increase in global temperatures cause will also increase water vapor concentrations and thus their warming effect, in a process known as water vapor feedback. It occurs because Clausius–Clapeyron relation establishes that more water vapor will be present per unit volume at elevated temperatures.[38] Thus, local atmospheric concentration of water vapor varies from less than 0.01% in extremely cold regions and up to 3% by mass in saturated air at about 32 °C.[39]
Global warming potential (GWP) and CO2 equivalents

Global Warming Potential (GWP) is an index to measure how much infrared thermal radiation a greenhouse gas would absorb over a given time frame after it has been added to the atmosphere (or emitted to the atmosphere). The GWP makes different greenhouse gases comparable with regard to their "effectiveness in causing radiative forcing".[40]: 2232 It is expressed as a multiple of the radiation that would be absorbed by the same mass of added carbon dioxide (CO2), which is taken as a reference gas. Therefore, the GWP has a value of 1 for CO2. For other gases it depends on how strongly the gas absorbs infrared thermal radiation, how quickly the gas leaves the atmosphere, and the time frame being considered.
For example, methane has a GWP over 20 years (GWP-20) of 81.2[41] meaning that, for example, a leak of a tonne of methane is equivalent to emitting 81.2 tonnes of carbon dioxide measured over 20 years. As methane has a much shorter atmospheric lifetime than carbon dioxide, its GWP is much less over longer time periods, with a GWP-100 of 27.9 and a GWP-500 of 7.95.[41]: 7SM-24
The carbon dioxide equivalent (CO2e or CO2eq or CO2-e or CO2-eq) can be calculated from the GWP. For any gas, it is the mass of CO2 that would warm the earth as much as the mass of that gas. Thus it provides a common scale for measuring the climate effects of different gases. It is calculated as GWP times mass of the other gas.List of all greenhouse gases

The contribution of each gas to the enhanced greenhouse effect is determined by the characteristics of that gas, its abundance, and any indirect effects it may cause. For example, the direct radiative effect of a mass of methane is about 84 times stronger than the same mass of carbon dioxide over a 20-year time frame.[45] Since the 1980s, greenhouse gas forcing contributions (relative to year 1750) are also estimated with high accuracy using IPCC-recommended expressions derived from radiative transfer models.[46]
The concentration of a greenhouse gas is typically measured in parts per million (ppm) or parts per billion (ppb) by volume. A CO2 concentration of 420 ppm means that 420 out of every million air molecules is a CO2 molecule. The first 30 ppm increase in CO2 concentrations took place in about 200 years, from the start of the Industrial Revolution to 1958; however the next 90 ppm increase took place within 56 years, from 1958 to 2014.[12][47][48] Similarly, the average annual increase in the 1960s was only 37% of what it was in 2000 through 2007.[49]
Many observations are available online in a variety of Atmospheric Chemistry Observational Databases. The table below shows the most influential long-lived, well-mixed greenhouse gases, along with their tropospheric concentrations and direct radiative forcings, as identified by the Intergovernmental Panel on Climate Change (IPCC).[50] Abundances of these trace gases are regularly measured by atmospheric scientists from samples collected throughout the world.[51][52][53] It excludes water vapor because changes in its concentrations are calculated as a climate change feedback indirectly caused by changes in other greenhouse gases, as well as ozone, whose concentrations are only modified indirectly by various refrigerants that cause ozone depletion. Some short-lived gases (e.g. carbon monoxide, NOx) and aerosols (e.g. mineral dust or black carbon) are also excluded because of limited role and strong variation, alongwith minor refrigerants and other halogenated gases, which have been mass-produced in smaller quantities than those in the table.[50]: 731–738 and Annex III of the 2021 IPCC WG1 Report[54]: 4–9
| Species | Lifetime
(years) [50]: 731 |
100-yr | Mole Fraction a + Radiative forcing | Concentrations
up to year 2022 | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline
Year 1750 |
TAR[57]
Year 1998 |
AR4[58]
Year 2005 |
AR5[50]: 678
Year 2011 |
AR6[54]: 4–9
Year 2019 | ||||
| CO2 | 1 | 278 | 365 (1.46) | 379 (1.66) | 391 (1.82) | 410 (2.16) | 
| |
| CH4 | 12.4 | 28 | 700 | 1,745 (0.48) | 1,774 (0.48) | 1,801 (0.48) | 1866 (0.54) | 
|
| N2O | 121 | 265 | 270 | 314 (0.15) | 319 (0.16) | 324 (0.17) | 332 (0.21) | 
|
| CFC-11 | 45 | 4,660 | 0 | 268 (0.07) | 251 (0.063) | 238 (0.062) | 226 (0.066) | 
|
| CFC-12 | 100 | 10,200 | 0 | 533 (0.17) | 538 (0.17) | 528 (0.17) | 503 (0.18) | 
|
| CFC-13 | 640 | 13,900 | 0 | 4 (0.001) | – | 2.7 (0.0007) | 3.28 (0.0009) | cfc13 |
| CFC-113 | 85 | 6,490 | 0 | 84 (0.03) | 79 (0.024) | 74 (0.022) | 70 (0.021) | 
|
| CFC-114 | 190 | 7,710 | 0 | 15 (0.005) | – | – | 16 (0.005) | cfc114 |
| CFC-115 | 1,020 | 5,860 | 0 | 7 (0.001) | – | 8.37 (0.0017) | 8.67 (0.0021) | cfc115 |
| HCFC-22 | 11.9 | 5,280 | 0 | 132 (0.03) | 169 (0.033) | 213 (0.0447) | 247 (0.0528) | 
|
| HCFC-141b | 9.2 | 2,550 | 0 | 10 (0.001) | 18 (0.0025) | 21.4 (0.0034) | 24.4 (0.0039) | 
|
| HCFC-142b | 17.2 | 5,020 | 0 | 11 (0.002) | 15 (0.0031) | 21.2 (0.0040) | 22.3 (0.0043) | 
|
| CH3CCl3 | 5 | 160 | 0 | 69 (0.004) | 19 (0.0011) | 6.32 (0.0004) | 1.6 (0.0001) | 
|
| CCl4 | 26 | 1,730 | 0 | 102 (0.01) | 93 (0.012) | 85.8 (0.0146) | 78 (0.0129) | 
|
| HFC-23 | 222 | 12,400 | 0 | 14 (0.002) | 18 (0.0033) | 24 (0.0043) | 32.4 (0.0062) | 
|
| HFC-32 | 5.2 | 677 | 0 | – | – | 4.92 (0.0005) | 20 (0.0022) | 
|
| HFC-125 | 28.2 | 3,170 | 0 | – | 3.7 (0.0009) | 9.58 (0.0022) | 29.4 (0.0069) | 
|
| HFC-134a | 13.4 | 1,300 | 0 | 7.5 (0.001) | 35 (0.0055) | 62.7 (0.0100) | 107.6 (0.018) | 
|
| HFC-143a | 47.1 | 4,800 | 0 | – | – | 12.0 (0.0019) | 24 (0.0040) | 
|
| HFC-152a | 1.5 | 138 | 0 | 0.5 (0.0000) | 3.9 (0.0004) | 6.4 (0.0006) | 7.1 (0.0007) | 
|
| CF4 (PFC-14) | 50,000 | 6,630 | 40 | 80 (0.003) | 74 (0.0034) | 79 (0.0040) | 85.5 (0.0051) | 
|
| C2F6 (PFC-116) | 10,000 | 11,100 | 0 | 3 (0.001) | 2.9 (0.0008) | 4.16 (0.0010) | 4.85 (0.0013) | 
|
| SF6 | 3,200 | 23,500 | 0 | 4.2 (0.002) | 5.6 (0.0029) | 7.28 (0.0041) | 9.95 (0.0056) | 
|
| SO2F2 | 36 | 4,090 | 0 | – | – | 1.71 (0.0003) | 2.5 (0.0005) | 
|
| NF3 | 500 | 16,100 | 0 | – | – | 0.9 (0.0002) | 2.05 (0.0004) | 
|
a Mole fractions: μmol/mol = ppm = parts per million (106); nmol/mol = ppb = parts per billion (109); pmol/mol = ppt = parts per trillion (1012).
A The IPCC states that "no single atmospheric lifetime can be given" for CO2.[50]: 731 This is mostly due to the rapid growth and cumulative magnitude of the disturbances to Earth's carbon cycle by the geologic extraction and burning of fossil carbon.[59] As of year 2014, fossil CO2 emitted as a theoretical 10 to 100 GtC pulse on top of the existing atmospheric concentration was expected to be 50% removed by land vegetation and ocean sinks in less than about a century, as based on the projections of coupled models referenced in the AR5 assessment.[60] A substantial fraction (20–35%) was also projected to remain in the atmosphere for centuries to millennia, where fractional persistence increases with pulse size.[61][62]
B Values are relative to year 1750. AR6 reports the effective radiative forcing which includes effects of rapid adjustments in the atmosphere and at the surface.[63]
Factors affecting concentrations
Atmospheric concentrations are determined by the balance between sources (emissions of the gas from human activities and natural systems) and sinks (the removal of the gas from the atmosphere by conversion to a different chemical compound or absorption by bodies of water).[64]: 512
Zdroj:https://en.wikipedia.org?pojem=Greenhouse-gas
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk
