A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tetrachloromethane | |||
| Other names
Benzinoform
carbon(IV) chloride carbon tet Carboneum Tetrachloratum / Carbonei tetrachloridum Carboneum Chloratum / Carbonei chlorurum chloride of carbon Freon-10 Halon-104 methane tetrachloride methyl tetrachloride Necatorina perchloromethane Refrigerant-10 Tetrachloretum Carbonicum Tetrachlorocarbon Tetraform Tetrasol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | CTC, TCM, PCM, R-10 | ||
| 1098295 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.239 | ||
| EC Number |
| ||
| 2347 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1846 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CCl4 | |||
| Molar mass | 153.81 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | chloroform-like odor | ||
| Density |
| ||
| Melting point | −22.92 °C (−9.26 °F; 250.23 K) | ||
| Boiling point | 76.72 °C (170.10 °F; 349.87 K) | ||
| |||
| Solubility | Soluble in alcohol, ether, chloroform, benzene, naphtha, CS2, formic acid | ||
| log P | 2.64 | ||
| Vapor pressure | 11.94 kPa at 20 °C | ||
Henry's law
constant (kH) |
2.76×10−2 atm·m3/mol | ||
| −66.60×10−6 cm3/mol | |||
| Thermal conductivity | 0.1036 W/m·K (300 K)[1] | ||
Refractive index (nD)
|
1.4607 | ||
| Viscosity | 0.86 mPa·s[2] | ||
| 0 D | |||
| Structure | |||
| Monoclinic | |||
| Tetragonal | |||
| Tetrahedral | |||
| 0 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
132.6 J/mol·K | ||
Std molar
entropy (S⦵298) |
214.39 J/mol·K | ||
Std enthalpy of
formation (ΔfH⦵298) |
−95.6 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
−87.34 kJ/mol[3] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
extremely toxic to the liver and kidneys, potential occupational carcinogen, harmful to the ozone layer | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H301, H302, H311, H331, H351, H372, H412, H420 | |||
| P201, P202, P260, P261, P264, P270, P271, P273, P280, P281, P301+P310, P302+P352, P304+P340, P308+P313, P311, P312, P314, P321, P322, P330, P361, P363, P403+P233, P405, P501, P502 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
7749 mg/kg (oral, mouse); 5760 mg/kg (oral, rabbit); 2350 mg/kg (oral, rat)[5] | ||
LC50 (median concentration)
|
| ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 10 ppm C 25 ppm 200 ppm (5-minute maximum peak in any 4 hours)[4] | ||
REL (Recommended)
|
Ca ST 2 ppm (12.6 mg/m3) [4] | ||
IDLH (Immediate danger)
|
200 ppm[4] | ||
| Safety data sheet (SDS) | ICSC 0024 | ||
| Related compounds | |||
Other anions
|
Carbon tetrafluoride Carbon tetrabromide Carbon tetraiodide | ||
Other cations
|
Silicon tetrachloride Germanium tetrachloride Tin tetrachloride Lead tetrachloride | ||
Related chloromethanes
|
Chloromethane Dichloromethane Trichloromethane | ||
| Supplementary data page | |||
| Carbon tetrachloride (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also recognised by the IUPAC) is a chemical compound with the chemical formula CCl4. It is a non-flammable, dense, colourless liquid with a "sweet" chloroform-like odour that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal.
Tradenames include: Carbon-Tet, Katharin (Germany, 1890s),[7] Benzinoform, Carbona and Thawpit in the cleaning industry, Halon-104 in firefighting, Refrigerant-10 in HVACR, and Necatorina and Seretin as a medication.
Properties
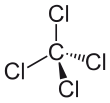
In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane. As a solvent, it is well suited to dissolving other non-polar compounds such as fats and oils. It can also dissolve iodine. It is volatile, giving off vapors with an odor characteristic of other chlorinated solvents, somewhat similar to the tetrachloroethylene odor reminiscent of dry cleaners' shops.
Solid tetrachloromethane has two polymorphs: crystalline II below −47.5 °C (225.6 K) and crystalline I above −47.5 °C.[8] At −47.3 °C it has monoclinic crystal structure with space group C2/c and lattice constants a = 20.3, b = 11.6, c = 19.9 (.10−1 nm), β = 111°.[9]
With a specific gravity greater than 1, carbon tetrachloride will be present as a dense nonaqueous phase liquid if sufficient quantities are spilt in the environment.
Reactions
Despite being generally inert, carbon tetrachloride can undergo various reactions. Hydrogen or an acid in the presence of an iron catalyst can reduce carbon tetrachloride to chloroform, dichloromethane, chloromethane and even methane.[10] When its vapours are passed through a red-hot tube, carbon tetrachloride dechlorinates to tetrachloroethylene and hexachloroethane.[11]
Carbon tetrachloride, when treated with HF, gives various compounds such as trichlorofluoromethane (R-11), dichlorodifluoromethane (R-12), chlorotrifluoromethane (R-13) and carbon tetrafluoride with HCl as the by-product:
This was once one of the main uses of carbon tetrachloride, as R-11 and R-12 were widely used as refrigerants.
An alcohol solution of potassium hydroxide decomposes it to potassium chloride and potassium carbonate in water:[12]
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk