A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

This article duplicates the scope of other articles, specifically Electrolytic capacitor. (May 2020) |
Aluminum electrolytic capacitors are (usually) polarized electrolytic capacitors whose anode electrode (+) is made of a pure aluminum foil with an etched surface. The aluminum forms a very thin insulating layer of aluminum oxide by anodization that acts as the dielectric of the capacitor. A non-solid electrolyte covers the rough surface of the oxide layer, serving in principle as the second electrode (cathode) (-) of the capacitor. A second aluminum foil called "cathode foil" contacts the electrolyte and serves as the electrical connection to the negative terminal of the capacitor.
Aluminum electrolytic capacitors are divided into three subfamilies by electrolyte type:
- non-solid (liquid, wet) aluminum electrolytic capacitors,
- solid manganese dioxide aluminum electrolytic capacitors, and
- solid polymer aluminum electrolytic capacitors.
Aluminum electrolytic capacitors with non-solid electrolyte are the most inexpensive type and also those with widest range of sizes, capacitance and voltage values. They are made with capacitance values from 0.1 μF up to 2,700,000 μF (2.7 F),[1] and voltage ratings ranging from 4 V up to 630 V.[2] The liquid electrolyte provides oxygen for re-forming or "self-healing" of the dielectric oxide layer. However, it can evaporate through a temperature-dependent drying-out process, which causes electrical parameters to drift, limiting the service life time of the capacitors.
Due to their relatively high capacitance values aluminum electrolytic capacitors have low impedance values even at lower frequencies like mains frequency. They are typically used in power supplies, switched-mode power supplies and DC-DC converters for smoothing and buffering rectified DC voltages in many electronic devices as well as in industrial power supplies and frequency converters as DC link capacitors for drives, inverters for photovoltaic, and converters in wind power plants. Special types are used for energy storage, for example in photoflash or strobe applications or for signal coupling in audio applications.
Aluminum electrolytic capacitors are polarized capacitors because of their anodization principle. They can only be operated with DC voltage applied with the correct polarity. Operating the capacitor with the wrong polarity, or with AC voltage, leads to a short circuit which can destroy the component. The exception is the bipolar or non-polar aluminum electrolytic capacitor, which has a back-to-back configuration of two anodes in a single case, and which can be safely used in AC applications.
Basic information
Oxide layer

Electrolytic capacitors use a chemical feature of some special metals, earlier called "valve metals". Applying a positive voltage to the anode material in an electrolytic bath forms an insulating oxide layer with a thickness corresponding to the applied voltage. This oxide layer acts as the dielectric in an electrolytic capacitor. The properties of this aluminum oxide layer compared with tantalum pentoxide dielectric layer are given in the following table:
| Anode- material |
Dielectric | Oxide structure |
Relative permittivity |
Breakdown voltage (V/μm) |
Electric layer thickness (nm/V) |
|---|---|---|---|---|---|
| Aluminum | Aluminium oxide Al2O3 | amorphous | 9.6 | 710 | 1.4 |
| crystalline | 11.6...14.2[4] | 800...1000[5] | 1.25...1.0 | ||
| Tantalum | Tantalum pentoxide Ta2O5 | amorphous | 27 | 625 | 1.6 |
After forming a dielectric oxide on the rough anode structures, a counter-electrode has to match the rough insulating oxide surface. This is provided by the electrolyte, which acts as the cathode electrode of an electrolytic capacitor. Electrolytes may be "non-solid" (wet, liquid) or "solid". Non-solid electrolytes, as a liquid medium that has an ion conductivity caused by moving ions, are relatively insensitive to voltage spikes or current surges. Solid electrolytes have an electron conductivity, which makes solid electrolytic capacitors sensitive to voltages spikes or current surges.
The anodic generated insulating oxide layer is destroyed if the polarity of the applied voltage changes.

Every electrolytic capacitor in principle forms a "plate capacitor" whose capacitance is greater the larger the electrode area A and the permittivity ε, and the thinner the thickness (d) of the dielectric.
The capacitance is proportional to the product of the area of one plate multiplied with the permittivity, divided by the thickness of the dielectric.
Electrolytic capacitors obtain their large capacitance values by a large area and small dielectric thickness. The dielectric thickness of electrolytic capacitors is very thin, in the range of nanometers per volt, but the voltage strengths of these oxide layers are quite high. All etched or sintered anodes have a much higher surface compared to a smooth surface of the same area. This increases the capacitance value by a factor of up to 200 for aluminum electrolytic capacitors.[6][7]
Construction of non-solid aluminum electrolytic capacitors
- Basic construction of aluminum electrolytic capacitors with non-solid electrolytes
-
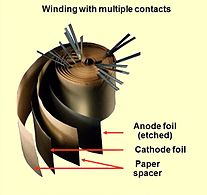
Opened winding of a capacitor with multiple connected foils
-
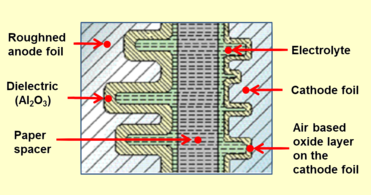
Closeup cross-section of an aluminum electrolytic capacitor design, showing capacitor anode foil with oxide layer, paper spacer soaked with electrolyte, and cathode foil
-
Construction of a typical single-ended aluminum electrolytic capacitor with non-solid electrolyte
An aluminum electrolytic capacitor with a non-solid electrolyte always consists of two aluminum foils separated mechanically by a spacer, mostly paper, which is saturated with a liquid or gel-like electrolyte. One of the aluminum foils, the anode, is etched (roughened) to increase the surface and oxidized (formed). The second aluminum foil, called the "cathode foil", serves to make electrical contact with the electrolyte. A paper spacer mechanically separates the foils to avoid direct metallic contact. Both foils and the spacer are wound and the winding is impregnated with liquid electrolyte. The electrolyte, which serves as cathode of the capacitor, covers the etched rough structure of the oxide layer on the anode perfectly and makes the increased anode surface effectual. After impregnation the impregnated winding is mounted in an aluminum case and sealed.
By design, a non-solid aluminum electrolytic capacitor has a second aluminum foil, the so-called cathode foil, for contacting the electrolyte. This structure of an aluminum electrolytic capacitor results in a characteristic result because the second aluminum (cathode) foil is also covered with an insulating oxide layer naturally formed by air. Therefore, the construction of the electrolytic capacitor consists of two single series-connected capacitors with capacitance CA of the anode and capacitance CK of the cathode. The total capacitance of the capacitor Ce-cap is thus obtained from the formula of the series connection of two capacitors:
It follows that the total capacitance of the capacitor Ce-cap is mainly determined by the anode capacitance CA when the cathode capacitance CK is very large compared with the anode capacitance CA. This requirement is given when the cathode capacitance CK is approximately 10 times higher than the anode capacitance CA. This can be easily achieved because the natural oxide layer on a cathode surface has a voltage proof of approximately 1.5 V and is therefore very thin.
Comparison of non-solid and solid types
Although the present article only refers in essence to aluminum electrolytic capacitors with non-solid electrolyte, an overview of the different types of aluminum electrolytic capacitors is given here in order to highlight the differences. Aluminum electrolytic capacitors are divided into two sub-types depending on whether they make use of liquid or solid electrolyte systems. Because the different electrolyte systems can be constructed with a variety of different materials, they include further sub-types.
- Aluminum electrolytic capacitors with non-solid electrolyte
- may use a liquid electrolyte based on ethylene glycol and boric acid, so-called "borax" electrolytes, or
- based on organic solvents, such as DMF, DMA, GBL, or
- based on high water containing solvents, for so-called "low impedance", "low ESR" or "high ripple current" capacitors
- Aluminum electrolytic capacitors with solid electrolyte
- have a solid manganese dioxide electrolyte, see solid aluminum capacitor (SAL), or
- a solid polymer electrolyte, see polymer aluminum electrolytic capacitor, or
- hybrid electrolytes, with both a solid polymer and a liquid, see also polymer aluminum electrolytic capacitor
- Principle design differences of the different aluminum electrolytic capacitor sub-types
-
Al-e-cap with non-solid electrolyte
-
Al-e-cap with solid manganese oxide electrolyte, graphite/silver cathode connection
-
Al-e-cap with polymer electrolyte
-
Al-e-cap with polymer electrolyte, graphite/silver cathode connection
-
Al-e-cap with polymer and non-solid electrolyte (Hybrid polymer)
Description of the materials
- 1: Anode foil, 2: Anode oxide layer (dielectric), 3: Cathode foil, 4: Cathode oxide layer, 5: Non-solid electrolyte, 6: Paper spacer soaked with electrolyte, either non-solid or polymer, 7: Conducting polymer, 8: Manganese oxide (MnO2), 9: Graphite, 10: Silver
The following table shows an overview over the main characteristics of the different types of aluminum electrolytic capacitors.
| Electrolyte | Capacitance range |
Rated voltage range |
Typical ESR 1) 100 kHz, 20 °C |
Typical ripple current 1) 100 kHz, 105 °C |
Leakage current 1) after 2 minutes at 10 V |
|---|---|---|---|---|---|
| Non-solid borax or organic | 0.1 μF–2.7 F | 4–630 V | 800 mΩ | 130 mA | < 10 μA |
| Non-solid water-based |
1–18000 μF | 4–100 V | 360 mΩ | 240 mA | 10 μA |
| Solid manganese dioxide |
0.1–1500 μF | 6.3–40 V[8] | 400 mΩ | 620 mA | 12 μA |
| Solid conducting polymer |
2.2–2700 μF | 2–125 V[9] | 25 mΩ | 2.5 A | 240 μA |
| Solid and non-solid hybrid electrolyte |
6.8–1000 μF | 6.3–125 V[10] | 40 mΩ | 1.5 A | 100 μA |
1) Values for a typical capacitor with 100 μF/10–16 V
Aluminum electrolytic capacitors with non-solid electrolyte are the best known and most widely used electrolytic capacitors. These components can be found on almost all boards of electronic equipment. They are characterized by particularly inexpensive and easy to process base materials.
Aluminum capacitors with liquid electrolytes based on borax or organic solvents have a large range of types and ratings. Capacitors with water-based electrolytes are often found in digital devices for mass production. Types with solid manganese dioxide electrolyte have served in the past as a "tantalum replacement". Polymer aluminum electrolytic capacitors with solid conductive polymer electrolytes are becoming increasingly important, especially in devices with a flat design, such as tablet PCs and flat panel displays. Electrolytic capacitors with hybrid electrolytes are relatively new on the market. With their hybrid electrolyte system, they combine the improved conductivity of the polymer with the advantage of liquid electrolytes for better self-healing properties of the oxide layer, so that the capacitors have the advantages of both low ESR and low leakage current.
Materials
Anode



The basic material of the anode for aluminum electrolytic capacitors is a foil with a thickness of ~ 20–100 μm made of aluminum with a high purity of at least 99.99%.[7][11] This is etched (roughened) in an electrochemical process to increase the effective electrode surface.[12] By etching the surface of the anode, depending on the required rated voltage, the surface area can be increased by a factor of approximately 200 with respect to a smooth surface.[7]
After etching the aluminum anode the roughed surface is "anodic oxidized" or "formed". An electrically insulating oxide layer Al2O3 is thereby formed on the aluminum surface by application of a current in correct polarity if it is inserted in an electrolytic bath. This oxide layer is the capacitor dielectric.
This process of oxide formation is carried out in two reaction steps whereby the oxygen for this reaction has to come from the electrolyte.[13] First, a strongly exothermic reaction transforms the metallic aluminum (Al) into aluminum hydroxide, Al(OH)3:
- 2 Al + 6 H2O → 2 Al(OH)3 + 3 H2 ↑
This reaction is accelerated by a high electric field and high temperatures, and is accompanied by a pressure buildup in the capacitor housing caused by the released hydrogen gas. The gel-like aluminum hydroxide Al(OH)3, also called alumina trihydrate (ATH), is converted via a second reaction step (usually slowly over a few hours at room temperature, more rapidly in a few minutes at higher temperatures) into aluminum oxide, Al2O3:
- 2 Al(OH)3 → 2 AlO(OH) + 2 H2O → Al2O3 + 3 H2O
The aluminum oxide serves as dielectric and also protects the metallic aluminum against aggressive chemical reactions from the electrolyte. However, the converted layer of aluminum oxide is usually not homogeneous. It forms a complex multilayer structured laminate of amorphous, crystalline and porous crystalline aluminum oxide mostly covered with small residual parts of unconverted aluminum hydroxide. For this reason, in the formation of the anode foil, the oxide film is structured by a special chemical treatment so that either an amorphous oxide or a crystalline oxide is formed. The amorphous oxide variety yields higher mechanical and physical stability and fewer defects, thus increasing the long term stability and lowering the leakage current.

Amorphous oxide has a dielectric ratio of ~ 1.4 nm/V. Compared to crystalline aluminum oxide, which has a dielectric ratio of ~1.0 nm/V, the amorphous variety has a 40% lower capacitance at the same anode surface.[3] The disadvantage of crystalline oxide is its greater sensitivity to tensile stress, which may lead to microcracks when subjected to mechanical (winding) or thermal (soldering) stressors during the post-forming processes.
The various properties of oxide structures affect the subsequent characteristics of the electrolytic capacitors. Anode foils with amorphous oxide are primarily used for electrolytic capacitors with stable long-life characteristics, for capacitors with low leakage current values, and for e-caps with rated voltages up to about 100 volts. Capacitors with higher voltages, for example photoflash capacitors, usually containing anode foils with crystalline oxide.[14]
Because the thickness of the effective dielectric is proportional to the forming voltage, the dielectric thickness can be tailored to the rated voltage of the capacitor. For example, for low voltage types a 10 V electrolytic capacitor has a dielectric thickness of only about 0.014 μm, a 100 V electrolytic capacitor of only about 0.14 μm. Thus, the dielectric strength also influences the size of the capacitor. However, due to standardized safety margins the actual forming voltage of electrolytic capacitors is higher than the rated voltage of the component.
Aluminum anode foils are manufactured as so-called "mother rolls" of about 500 mm in width. They are pre-formed for the desired rated voltage and with the desired oxide layer structure. To produce the capacitors, the anode widths and lengths, as required for a capacitor, have to be cut from the mother roll.[15]
Cathode

The second aluminum foil in the electrolytic capacitor, called the "cathode foil", serves to make electrical contact with the electrolyte. This foil has a somewhat lower degree of purity, about 99.8%. It is always provided with a very thin oxide layer, which arises from the contact of the aluminum surface with the air in a natural way. In order to reduce the contact resistance to the electrolyte and to make it difficult for oxide formation during discharging, the cathode foil is alloyed with metals such as copper, silicon, or titanium. The cathode foil is also etched to enlarge the surface.
Because of the extremely thin oxide layer, which corresponds to a voltage proof of about 1.5 V, their specific capacitance is, however, much higher than that of anode foils.[7] To justify the need for a large surface capacitance of the cathode foil see the section on charge/discharge stability below.
The cathode foils, as the anode foils, are manufactured as so-called "mother rolls", from which widths and lengths are cut off, as required, for capacitor production.
Electrolyte
The electrolytic capacitor got its name from the electrolyte, the conductive liquid inside the capacitor. As a liquid it can be adapted to the porous structure of the anode and the grown oxide layer with the same shape and form as a "tailor-made" cathode. An electrolyte always consists of a mixture of solvents and additives to meet given requirements. The main electrical property of the electrolyte is its conductivity, which is physically an ion-conductivity in liquids. In addition to the good conductivity of operating electrolytes, various other requirements are, among other things, chemical stability, high flash point, chemical compatibility with aluminum, low viscosity, minimal negative environmental impact and low cost. The electrolyte should also provide oxygen for forming and self-healing processes, and all this within a temperature range as wide as possible. This diversity of requirements for the liquid electrolyte results in a wide variety of proprietary solutions.[16][17]
The electrolytic systems used today can be roughly summarized into three main groups:
- Electrolytes based on ethylene glycol and boric acid. In these so-called glycol or borax electrolyte an unwanted chemical crystal water reaction occurs according to the scheme: "acid + alcohol" gives "ester + water". These borax electrolytes are standard electrolytes, long in use, and with a water content between 5 and 20%. They work at a maximum temperature of 85 °C or 105 °C in the entire voltage range up to 600 V. Even with these capacitors, the aggressiveness of the water must be prevented by appropriate measures.[18]
- Almost anhydrous electrolytes based on organic solvents, such as dimethylformamide (DMF), dimethylacetamide (DMA), or γ-butyrolactone (GBL). These capacitors with organic solvent electrolytes are suitable for temperature ranges from 105 °C, 125 °C or 150 °C, have low leakage current values and have very good long-term capacitor behavior.
- Water based electrolytes with high water content, up to 70% water for so-called "low-impedance", "low-ESR" or "high-ripple-current" electrolytic capacitors with rated voltages up to 100 V[19] for low-cost mass-market applications. The aggressiveness of the water for aluminum must be prevented with suitable additives.[20]
Since the amount of liquid electrolyte during the operating time of the capacitors decreases over time through self-healing and by diffusion through the seal, the electrical parameters of the capacitors may be adversely affected, limiting the service life or lifetime of "wet" electrolytic capacitors, see the section on lifetime below.
Separator
The anode and cathode foils must be protected from direct contact with each other because such contact, even at relatively low voltages, may lead to a short circuit. In case of direct contact of both foils the oxide layer on the anode surface gives no protection. A spacer or separator made of a special highly absorbent paper with high purity protects the two metal foils from direct contact. This capacitor paper also serves as a reservoir for the electrolyte to extend the lifetime of the capacitor.
The thickness of the spacer depends on the rated voltage of the electrolytic capacitor. It is up to 100 V between 30 and 75 μm.[21] For higher voltages, several layers of paper (duplex paper) are used to increase the breakdown strength.
Encapsulation

The encapsulation of aluminum electrolytic capacitors is also made of aluminum in order to avoid galvanic reactions, normally with an aluminum case (can, tub). For radial electrolytic capacitors it is connected across the electrolyte with a non-defined resistance to the cathode (ground). For axial electrolytic capacitors, however, the housing is specifically designed with a direct contact to the cathode.
In case of a malfunction, overload or wrong polarity operating inside the electrolytic capacitor housing, substantial gas pressure can arise. The tubs are designed to open a pressure relief vent and release high pressure gas, including parts of the electrolyte. This vent protects against bursting, explosion or fly away of the metal tub.
For smaller housings the pressure relief vent is carved in the bottom or the notch of the tub. Larger capacitors like screw-terminal capacitors have a lockable overpressure vent and must be mounted in an upright position.
Sealing
The sealing materials of aluminum electrolytic capacitors depend on the different styles. For larger screw-terminal and snap-in capacitors the sealing washer is made of a plastic material. Axial electrolytic capacitors usually have a sealing washer made of phenolic resin laminated with a layer of rubber. Radial electrolytic capacitors use a rubber plug with a very dense structure. All sealing materials must be inert to the chemical parts of the electrolyte and may not contain soluble compounds that could lead to contamination of the electrolyte. To avoid leakage, the electrolyte must not be aggressive to the sealing material.
Production

The production process starts with mother rolls. First, the etched, roughened and pre-formed anode foil on the mother roll as well as the spacer paper and the cathode foil are cut to the required width.[11][12] The foils are fed to an automatic winder, which makes a wound section in a consecutive operation involving three sequential steps: terminal welding, winding, and length cutting. In the next production step the wound section fixed at the lead out terminals is soaked with electrolyte under vacuum impregnation. The impregnated winding is then built into an aluminum case, provided with a rubber sealing disc, and mechanically tightly sealed by curling. Thereafter, the capacitor is provided with an insulating shrink sleeve film. This optically ready capacitor is then contacted at rated voltage in a high temperature post-forming device for healing all the dielectric defects resulting from the cutting and winding procedure. After post-forming, a 100% final measurement of capacitance, leakage current, and impedance takes place. Taping closes the manufacturing process; the capacitors are ready for delivery.
Styles
- Different styles of non-solid aluminum electrolytic capacitors
Aluminum electrolytic capacitors with non-solid electrolyte are available in different styles, see pictures above from left to right:
- SMDs (V-chip) for surface mounting on printed circuit boards or substrates
- Radial lead terminals (single ended) for vertical mounting on printed circuit boards
- Axial lead terminals for horizontal THT mounting on printed circuit boards
- Radial pin terminals (snap-in) for power applications
- Large screw terminals for power applications
History

In 1875, French researcher Eugène Ducretet discovered that certain "valve metals" (aluminum and others) can form an oxide layer that blocks an electric current from flowing in one direction but allows it to flow in the reverse direction.
Karol Pollak, a producer of accumulators, found out that the oxide layer on an aluminum anode remained stable in a neutral or alkaline electrolyte, even when the power was switched off. In 1896 he obtained a patent for an Electric liquid capacitor with aluminum electrodes (de: Elektrischer Flüssigkeitskondensator mit Aluminiumelektroden) based on the idea of using the oxide layer in a polarized capacitor in combination with a neutral or slightly alkaline electrolyte.[22]
The first electrolytic capacitors realized industrially consisted of a metallic box used as cathode, filled with a borax electrolyte dissolved in water, in which a folded aluminum anode plate was inserted. Applying a DC voltage from outside, an oxide layer was formed on the surface of the anode. The advantage of these capacitors was that they were significantly smaller and cheaper than all other capacitors at this time with respect to realized capacitance value. This construction with different styles of anode construction but with a case as cathode and a container as the electrolyte was used up to the 1930s and was called a "wet" electrolytic capacitor, referring to its high water content.

The first common application of wet aluminum electrolytic capacitors was in large telephone exchanges, to reduce relay hash (noise) on the 48 volt DC power supply. The development of AC-operated domestic radio receivers in the late 1920s created a demand for large-capacitance (for the time) and high-voltage capacitors for the valve amplifier technique, typically at least 4 microfarads and rated at around 500 volts DC. Waxed paper and oiled silk film capacitors were available, but devices with that order of capacitance and voltage rating were bulky and prohibitively expensive.

The ancestor of the modern electrolytic capacitor was patented by Samuel Ruben in 1925,[23][24] who teamed with Philip Mallory, the founder of the battery company that is now known as Duracell International. Ruben's idea adopted the stacked construction of a silver mica capacitor. He introduced a separate second foil to contact the electrolyte adjacent the anode foil instead of using the electrolyte-filled container as the cathode of the capacitor. The stacked second foil got its own terminal additional to the anode terminal and the container had no longer an electrical function. This type of electrolytic capacitor with one anode foil separated from a cathode foil by a liquid or gel-like electrolyte of a non-aqueous nature, which is therefore dry in the sense of having a very low water content, became known as the "dry" type of electrolytic capacitor.[25] This invention, together with the invention of wound foils separated with a paper spacer 1927 by A. Eckel, Hydra-Werke (Germany),[26] reduced the size and the price significantly, which helped make the new radios affordable for a broader group of customers.[25]
William Dubilier, whose first patent for electrolytic capacitors was filed in 1928,[27] industrialized the new ideas for electrolytic capacitors and started large-scale commercial production in 1931 in the Cornell-Dubilier (CD) factory in Plainfield, New Jersey.[25] At the same time in Berlin, Germany, the "Hydra-Werke", an AEG company, started the production of electrolytic capacitors in large quantities.
Already in his patent application of 1886, Pollak wrote that the capacitance of the capacitor increased if the surface of the anode foil was roughened. A number of methods have since been developed for roughening the anode surface, mechanical methods like sand blasting or scratching, and chemical etching with acids and acid salts forced by high currents.[28] Some of these methods were developed in the CD factory between 1931 and 1938. Today (2014), electro-chemically etching of low voltage foils can achieve up to a 200 fold increase in surface area compared to a smooth surface.[6][7] Progress relating to the etching process is the reason for the ongoing reduction in the dimensions of aluminum electrolytic capacitors over the past decades.

The period after World War II is associated with a rapid development in radio and television technology as well as in industrial applications, which had great influence on production quantities but also on styles, sizes and series diversification of electrolytic capacitors. New electrolytes based on organic liquids reduced leakage currents and ESR, broadened temperature ranges and increased lifetimes. Corrosion phenomena caused by chlorine and water could be avoided by a higher purity manufacturing processes and by using additives in the electrolytes.
The development of tantalum electrolytic capacitors in the early 1950s[29][30] with manganese dioxide as solid electrolyte, which has a 10 times better conductivity than all other types of non-solid electrolytes, also influenced the development of aluminum electrolytic capacitors. In 1964 the first aluminum electrolytic capacitors with solid electrolyte (Solid aluminum capacitor (SAL)) appeared on the market, developed by Philips.[31]
The decades from 1970 to 1990 were marked by the development of various new professional aluminum electrolytic capacitor series with f. e. very low leakage currents or with long life characteristics or for higher temperatures up to 125 °C, which were specifically suited to certain industrial applications.[32] The great diversity of the many series of aluminum electrolytic capacitors with non-solid electrolytes up to now (2014) is an indicator of the adaptability of the capacitors to meet different industrial requirements.

In 1983 a further reduction of the ESR was achieved by Sanyo with its "OS-CON" aluminum electrolytic capacitors. These capacitors use as solid organic conductor the charge transfer salt TTF-TCNQ (tetracyanoquinodimethane), which provided an improvement in conductivity by a factor of 10 with respect to the manganese dioxide electrolyte.
The ESR values of TCNQ-capacitors were significantly reduced by the discovery of conducting polymers by Alan J. Heeger, Alan MacDiarmid and Hideki Shirakawa.[33] The conductivity of conductive polymers such as polypyrrole or PEDOT[34] are better than that of TCNQ by a factor of 100 to 500, and are close to the conductivity of metals. In 1991 Panasonic put its "SP-Cap",[35] a polymer aluminum electrolytic capacitor, on the market. These electrolytic capacitors with polymer electrolytes achieved ESR values low enough to compete with ceramic multilayer capacitors (MLCCs). They were still less expensive than tantalum capacitors and were a short time later used in devices with a flat design, such as laptops and cell phones.
New water-based electrolytes were developed in Japan from the mid-1980s with the goal of reducing ESR for inexpensive non-solid electrolytic capacitors. Water is inexpensive, an effective solvent for electrolytes, and significantly improves the conductivity of the electrolyte.
Japanese manufacturer Rubycon was a leader in the development of new water-based electrolyte systems with enhanced conductivity in the late 1990s.[19] The new series of non-solid capacitors with water-based electrolyte was called in the data sheets "Low-ESR", "Low-Impedance", "Ultra-Low-Impedance" or "High-Ripple Current" series.
From 2000 to 2005, a stolen recipe of such a water-based electrolyte missing important stabilizing substances[18][20] [36] led to the problem of mass-bursting capacitors in computers and power supplies, which became known as the "Capacitor Plague". In these capacitors the water reacts quite aggressively and even violently with aluminum, accompanied by strong heat and gas development in the capacitor, and often leads to the explosion of the capacitor.
Electrical parameters

The electrical characteristics of capacitors are harmonized by the international generic specification IEC 60384-1. In this standard, the electrical characteristics of capacitors are described by an idealized series-equivalent circuit with electrical components that model all ohmic losses, capacitive and inductive parameters of an electrolytic capacitor:
- C, the capacitance of the capacitor,
- RESR, the equivalent series resistance, which summarizes all ohmic losses of the capacitor, usually abbreviated as "ESR".
- LESL, the equivalent series inductance, which is the effective self-inductance of the capacitor, usually abbreviated as "ESL".
- Rleakage, the resistance that represents the leakage current
Capacitance standard values and tolerances
The basic unit of electrolytic capacitors capacitance is the microfarad (μF, or less correctly uF).
The capacitance value specified in manufacturers' data sheets is called the rated capacitance CR or nominal capacitance CN and is the value for which the capacitor has been designed. Standardized measuring conditions for electrolytic capacitors are an AC measurement with 0.5 V[clarification needed] at a frequency of 100/120 Hz and a temperature of 20 °C.[citation needed]
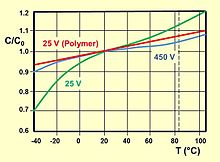
The capacitance value of an electrolytic capacitor depends on the measuring frequency and temperature. The value at a measuring frequency of 1 kHz is about 10% less than the 100/120 Hz value. Therefore, the capacitance values of electrolytic capacitors are not directly comparable and differ from those of film capacitors or ceramic capacitors, whose capacitance is measured at 1 kHz or higher.
Measured with an AC measuring method with 100/120 Hz the measured capacitance value is the closest value to the electrical charge stored in the capacitor. The stored charge is measured with a special discharge method and is called DC capacitance. The DC capacitance is about 10% higher than the 100/120 Hz AC capacitance. The DC capacitance is of interest for discharge applications like photoflash.
The percentage of allowed deviation of the measured capacitance from the rated value is called capacitance tolerance. Electrolytic capacitors are available in different tolerance series, whose values are specified in the E series specified in IEC 60063. For abbreviated marking in tight spaces, a letter code for each tolerance is specified in IEC 60062.
- rated capacitance, E3 series, tolerance ±20%, letter code "M"
- rated capacitance, E6 series, tolerance ±20%, letter code "M"
- rated capacitance, E12 series, tolerance ±10%, letter code "K"
The required capacitance tolerance is determined by the particular application. Electrolytic capacitors that are often used for filtering and bypassing capacitors do not need narrow tolerances because they are not used for accurate frequency applications, such as for oscillators.
Rated and category voltage

In IEC 60384-1 the allowed operating voltage is called the "rated voltage" UR or the "nominal voltage" UN. The rated voltage is the maximum DC voltage or peak pulse voltage that may be applied continuously at any temperature within the rated temperature range.
The voltage proof of electrolytic capacitors, which is directly proportional to the dielectric layer thickness,[6] decreases with increasing temperature. For some applications it is important to use a high temperature range. Lowering the voltage applied at a higher temperature maintains safety margins. For some capacitor types, therefore, the IEC standard specifies a second "temperature derated voltage" for a higher temperature range, the "category voltage" UC. The category voltage is the maximum DC voltage, peak pulse voltage or superimposed AC voltage that may be applied continuously to a capacitor at any temperature within the category temperature range.
Surge voltage
Aluminum electrolytic capacitors can be applied for a short time with an overvoltage, also called a surge voltage. The surge voltage indicates the maximum voltage value within the temperature range that may be applied during the lifetime at a frequency of 1000 cycles (with a dwell time of 30 seconds and a pause of 5 minutes and 30 seconds in each instance) without causing any visible damage to the capacitor or a capacitance change of more than 15%.
Usually, for capacitors with a rated voltage of ≤ 315 volts, the surge voltage is 1.15 times the rated voltage and for capacitors with a rated voltage exceeding 315 volts the surge voltage is 1.10 times the rated voltage.
Transient voltage
Aluminum electrolytic capacitors with non-solid electrolyte are relatively insensitive to high and short-term transient voltages higher than the surge voltage, if the frequency and the energy content of the transients is low. This ability depends on the rated voltage and component size. Low energy transient voltages lead to a voltage limitation similar to a zener diode.
The electrochemical oxide forming processes take place when voltage in correct polarity is applied and generates an additional oxide when transients arise. This formation is accompanied with heat and hydrogen gas generation. This is tolerable if the energy content of the transient is low. However, when a transient peak voltage causes an electric field strength that is too high for the dielectric, it can directly cause a short circuit. An unambiguous and general specification of tolerable transients or peak voltages is not possible. In every case transients arise, the application has to be carefully approved.
Electrolytic capacitors with solid electrolyte cannot withstand transients or peak voltages higher than the surge voltage. Transients for this type of electrolytic capacitor may destroy the component.
Zdroj:https://en.wikipedia.org?pojem=Aluminium_electrolytic_capacitorText je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk