
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
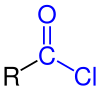
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids (R−C(=O)OH). A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Nomenclature
Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting -yl chloride for -ic acid. Thus:
- acetic acid (CH3COOH) → acetyl chloride (CH3COCl)
- benzoic acid (C6H5COOH) → benzoyl chloride (C6H5COCl)
- butyric acid (C3H7COOH) → butyryl chloride (C3H7COCl)
(Idiosyncratically, for some trivial names, -oyl chloride substitutes -ic acid. For example, pivalic acid becomes pivaloyl chloride and acrylic acid becomes acryloyl chloride. The names pivalyl chloride and acrylyl chloride are less commonly used, although they are arguably more logical.)
When other functional groups take priority, acyl chlorides are considered prefixes — chlorocarbonyl-:[1]
- acetic acid (CH3COOH) → (chlorocarbonyl)acetic acid (ClOCCH2COOH)
Properties
Lacking the ability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than similar carboxylic acids. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy reveals a band near 1750 cm−1.
The simplest stable acyl chloride is acetyl chloride; formyl chloride is not stable at room temperature, although it can be prepared at –60 °C or below.[2][3]
Acyl chlorides hydrolyze (react with water) to form the corresponding carboxylic acid and hydrochloric acid:

Synthesis
Industrial routes
The industrial route to acetyl chloride involves the reaction of acetic anhydride with hydrogen chloride:[5]
Propionyl chloride is produced by chlorination of propionic acid with phosgene:[6]
Benzoyl chloride is produced by the partial hydrolysis of benzotrichloride:[7]
Similarly, benzotrichlorides react with carboxylic acids to the acid chloride. This conversion is practiced for the reaction of 1,4-bis(trichloromethyl)benzene to give terephthaloyl chloride:
Laboratory methods: thionyl chloride
In the laboratory, acyl chlorides are generally prepared by treating carboxylic acids with thionyl chloride (SOCl2).[8] The reaction is catalyzed by dimethylformamide and other additives.[9][10]

Thionyl chloride[11] is a well-suited reagent as the by-products (HCl, SO2) are gases and residual thionyl chloride can be easily removed as a result of its low boiling point (76 °C).
Laboratory methods: phosphorus chlorides
Phosphorus trichloride (PCl3) is popular,[12] although excess reagent is required.[9] Phosphorus pentachloride (PCl5) is also effective,[13][14] but only one chloride is transferred:
Laboratory methods: oxalyl chloride
Another method involves the use of oxalyl chloride:
The reaction is catalysed by dimethylformamide (DMF), which reacts with oxalyl chloride to give the Vilsmeier reagent, an iminium intermediate that which reacts with the carboxylic acid to form a mixed imino-anhydride. This structure undergoes an acyl substitution with the liberated chloride, forming the acid anhydride and releasing regenerated molecule of DMF.[10] Relative to thionyl chloride, oxalyl chloride is more expensive but also a milder reagent and therefore more selective.
Other laboratory methods
Acid chlorides can be used as a chloride source.[15] Thus acetyl chloride can be distilled from a mixture of benzoyl chloride and acetic acid:[9]
Other methods that do not form HCl include the Appel reaction:[16]
Another is the use of cyanuric chloride:[17]
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk










