A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acetic acid[3] | |||
| Systematic IUPAC name
Ethanoic acid | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| Abbreviations | AcOH | ||
| 506007 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.528 | ||
| EC Number |
| ||
| E number | E260 (preservatives) | ||
| 1380 | |||
| KEGG | |||
| MeSH | Acetic+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2789 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH3COOH | |||
| Molar mass | 60.052 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Heavily vinegar-like | ||
| Density | 1.049 g/cm3 (liquid); 1.27 g/cm3 (solid) | ||
| Melting point | 16 to 17 °C; 61 to 62 °F; 289 to 290 K | ||
| Boiling point | 118 to 119 °C; 244 to 246 °F; 391 to 392 K | ||
| Miscible | |||
| log P | -0.28[4] | ||
| Vapor pressure | 1.54653947 kPa (20 °C) 11.6 mmHg (20 °C)[5] | ||
| Acidity (pKa) | 4.756 | ||
| Conjugate base | Acetate | ||
| -31.54·10−6 cm3/mol | |||
Refractive index (nD)
|
1.371 (VD = 18.19) | ||
| Viscosity | 1.22 mPa s 1.22 cP | ||
| 1.74 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
123.1 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
158.0 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
-483.88–483.16 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
-875.50–874.82 kJ/mol | ||
| Pharmacology | |||
| G01AD02 (WHO) S02AA10 (WHO) | |||
| Legal status |
| ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H226, H314 | |||
| P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 40 °C (104 °F; 313 K) | ||
| 427 °C (801 °F; 700 K) | |||
| Explosive limits | 4–16% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
3.31 g kg−1, oral (rat) | ||
LC50 (median concentration)
|
5620 ppm (mouse, 1 hr) 16000 ppm (rat, 4 hr)[7] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 10 ppm (25 mg/m3)[6] | ||
REL (Recommended)
|
TWA 10 ppm (25 mg/m3) ST 15 ppm (37 mg/m3)[6] | ||
IDLH (Immediate danger)
|
50 ppm[6] | ||
| Related compounds | |||
Related carboxylic acids
|
Formic acid Propionic acid | ||
Related compounds
|
Acetaldehyde Acetamide Acetic anhydride Chloroacetic acid Acetyl chloride Glycolic acid Ethyl acetate Potassium acetate Sodium acetate Thioacetic acid | ||
| Supplementary data page | |||
| Acetic acid (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Identifiers | |
| E number | E260 (preservatives) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.528 |
| Data page | |
| Acetic acid (data page) | |
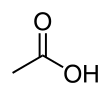
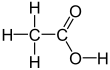
Acetic acid /əˈsiːtɪk/, systematically named ethanoic acid /ˌɛθəˈnoʊɪk/, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats.
The global demand for acetic acid is about 6.5 million metric tonnes per year (t/a), manufactured from methanol.[8] Its production and subsequent industrial use poses health hazards to workers, including incidental skin damage and chronic respiratory injuries from inhalation.[9]
Nomenclature
The trivial name "acetic acid" is the most commonly used and preferred IUPAC name. The systematic name "ethanoic acid", a valid IUPAC name, is constructed according to the substitutive nomenclature.[10] The name "acetic acid" derives from the Latin word for vinegar, "acetum", which is related to the word "acid" itself.
"Glacial acetic acid" is a name for water-free (anhydrous) acetic acid. Similar to the German name "Eisessig" ("ice vinegar"), the name comes from the solid ice-like crystals that form with agitation, slightly below room temperature at 16.6 °C (61.9 °F). Acetic acid can never be truly water-free in an atmosphere that contains water, so the presence of 0.1% water in glacial acetic acid lowers its melting point by 0.2 °C.[11]
A common symbol for acetic acid is AcOH (or HOAc), where Ac is the pseudoelement symbol representing the acetyl group CH3−C(=O)−; the conjugate base, acetate (CH3COO−), is thus represented as AcO−.[12] (The symbol Ac for the acetyl functional group is not to be confused with the symbol Ac for the element actinium; context prevents confusion among organic chemists). To better reflect its structure, acetic acid is often written as CH3−C(O)OH, CH3−C(=O)OH, CH3COOH, and CH3CO2H. In the context of acid–base reactions, the abbreviation HAc is sometimes used,[13] where Ac in this case is a symbol for acetate (rather than acetyl). Acetate is the ion resulting from loss of H+ from acetic acid. The name "acetate" can also refer to a salt containing this anion, or an ester of acetic acid.[14]
History
Vinegar was known early in civilization as the natural result of exposure of beer and wine to air because acetic acid-producing bacteria are present globally. The use of acetic acid in alchemy extends into the third century BC, when the Greek philosopher Theophrastus described how vinegar acted on metals to produce pigments useful in art, including white lead (lead carbonate) and verdigris, a green mixture of copper salts including copper(II) acetate. Ancient Romans boiled soured wine to produce a highly sweet syrup called sapa. Sapa that was produced in lead pots was rich in lead acetate, a sweet substance also called sugar of lead or sugar of Saturn, which contributed to lead poisoning among the Roman aristocracy.[15]
In the 16th-century German alchemist Andreas Libavius described the production of acetone from the dry distillation of lead acetate, ketonic decarboxylation. The presence of water in vinegar has such a profound effect on acetic acid's properties that for centuries chemists believed that glacial acetic acid and the acid found in vinegar were two different substances. French chemist Pierre Adet proved them identical.[15][16]

In 1845 German chemist Hermann Kolbe synthesised acetic acid from inorganic compounds for the first time. This reaction sequence consisted of chlorination of carbon disulfide to carbon tetrachloride, followed by pyrolysis to tetrachloroethylene and aqueous chlorination to trichloroacetic acid, and concluded with electrolytic reduction to acetic acid.[17]
By 1910, most glacial acetic acid was obtained from the pyroligneous liquor, a product of the distillation of wood. The acetic acid was isolated by treatment with milk of lime, and the resulting calcium acetate was then acidified with sulfuric acid to recover acetic acid. At that time, Germany was producing 10,000 tons of glacial acetic acid, around 30% of which was used for the manufacture of indigo dye.[15][18]
Because both methanol and carbon monoxide are commodity raw materials, methanol carbonylation long appeared to be attractive precursors to acetic acid. Henri Dreyfus at British Celanese developed a methanol carbonylation pilot plant as early as 1925.[19] However, a lack of practical materials that could contain the corrosive reaction mixture at the high pressures needed (200 atm or more) discouraged commercialization of these routes. The first commercial methanol carbonylation process, which used a cobalt catalyst, was developed by German chemical company BASF in 1963. In 1968, a rhodium-based catalyst (cis−[Rh(CO)2I2−) was discovered that could operate efficiently at lower pressure with almost no by-products. US chemical company Monsanto Company built the first plant using this catalyst in 1970, and rhodium-catalyzed methanol carbonylation became the dominant method of acetic acid production (see Monsanto process). In the late 1990s, BP Chemicals commercialised the Cativa catalyst ([Ir(CO)2I2−), which is promoted by iridium for greater efficiency.[20] Known as the Cativa process, the iridium-catalyzed production of glacial acetic acid is greener, and has largely supplanted the Monsanto process, often in the same production plants.[21]
Interstellar medium
Interstellar acetic acid was discovered in 1996 by a team led by David Mehringer[22] using the former Berkeley-Illinois-Maryland Association array at the Hat Creek Radio Observatory and the former Millimeter Array located at the Owens Valley Radio Observatory. It was first detected in the Sagittarius B2 North molecular cloud (also known as the Sgr B2 Large Molecule Heimat source). Acetic acid has the distinction of being the first molecule discovered in the interstellar medium using solely radio interferometers; in all previous ISM molecular discoveries made in the millimetre and centimetre wavelength regimes, single dish radio telescopes were at least partly responsible for the detections.[22]
Properties

Acidity
The hydrogen centre in the carboxyl group (−COOH) in carboxylic acids such as acetic acid can separate from the molecule by ionization:
- CH3COOH ⇌ CH3CO−2 + H+
Because of this release of the proton (H+), acetic acid has acidic character. Acetic acid is a weak monoprotic acid. In aqueous solution, it has a pKa value of 4.76.[23] Its conjugate base is acetate (CH3COO−). A 1.0 M solution (about the concentration of domestic vinegar) has a pH of 2.4, indicating that merely 0.4% of the acetic acid molecules are dissociated.[a] Only in very dilute (< 10−6 M) solution, acetic acid is >90% dissociated.[citation needed]

Structure
In solid acetic acid, the molecules form chains of individual molecules interconnected by hydrogen bonds.[24] In the vapour phase at 120 °C (248 °F), dimers can be detected. Dimers also occur in the liquid phase in dilute solutions with non-hydrogen-bonding solvents, and to a certain extent in pure acetic acid,[25] but are disrupted by hydrogen-bonding solvents. The dissociation enthalpy of the dimer is estimated at 65.0–66.0 kJ/mol, and the dissociation entropy at 154–157 J mol−1 K−1.[26] Other carboxylic acids engage in similar intermolecular hydrogen bonding interactions.[27]
Solvent properties
Liquid acetic acid is a hydrophilic (polar) protic solvent, similar to ethanol and water. With a relative static permittivity (dielectric constant) of 6.2, it dissolves not only polar compounds such as inorganic salts and sugars, but also non-polar compounds such as oils as well as polar solutes. It is miscible with polar and non-polar solvents such as water, chloroform, and hexane. With higher alkanes (starting with octane), acetic acid is not miscible at all compositions, and solubility of acetic acid in alkanes declines with longer n-alkanes.[28] The solvent and miscibility properties of acetic acid make it a useful industrial chemical, for example, as a solvent in the production of dimethyl terephthalate.[8]
Biochemistry
At physiological pHs, acetic acid is usually fully ionised to acetate.[29]
The acetyl group, formally derived from acetic acid, is fundamental to all forms of life. Typically, it is bound to coenzyme A by acetyl-CoA synthetase enzymes,[30] where it is central to the metabolism of carbohydrates and fats. Unlike longer-chain carboxylic acids (the fatty acids), acetic acid does not occur in natural triglycerides. Most of the aceate generated in cells for use in acetyl-CoA is synthesized directly from ethanol or pyruvate.[31] However, the artificial triglyceride triacetin (glycerine triacetate) is a common food additive and is found in cosmetics and topical medicines; this additive is metabolized to glycerol and acetic acid in the body.[32]
Acetic acid is produced and excreted by acetic acid bacteria, notably the genus Acetobacter and Clostridium acetobutylicum. These bacteria are found universally in foodstuffs, water, and soil, and acetic acid is produced naturally as fruits and other foods spoil. Acetic acid is also a component of the vaginal lubrication of humans and other primates, where it appears to serve as a mild antibacterial agent.[33]
Production

Acetic acid is produced industrially both synthetically and by bacterial fermentation. About 75% of acetic acid made for use in the chemical industry is made by the carbonylation of methanol, explained below.[8] The biological route accounts for only about 10% of world production, but it remains important for the production of vinegar because many food purity laws require vinegar used in foods to be of biological origin. Other processes are methyl formate isomerization, conversion of syngas to acetic acid, and gas phase oxidation of ethylene and ethanol.[34]
Acetic acid can be purified via fractional freezing using an ice bath. The water and other impurities will remain liquid while the acetic acid will precipitate out. As of 2003–2005, total worldwide production of virgin acetic acid[b] was estimated at 5 Mt/a (million tonnes per year), approximately half of which was produced in the United States. European production was approximately 1 Mt/a and declining, while Japanese production was 0.7 Mt/a. Another 1.5 Mt were recycled each year, bringing the total world market to 6.5 Mt/a.[35][36] Since then, the global production has increased from 10.7 Mt/a in 2010[37] to 17.88 Mt/a in 2023.[38] The two biggest producers of virgin acetic acid are Celanese and BP Chemicals. Other major producers include Millennium Chemicals, Sterling Chemicals, Samsung, Eastman, and Svensk Etanolkemi.[39]
Methanol carbonylation
Most acetic acid is produced by methanol carbonylation. In this process, methanol and carbon monoxide react to produce acetic acid according to the equation:
The process involves iodomethane as an intermediate, and occurs in three steps. A metal carbonyl catalyst is needed for the carbonylation (step 2).[34]
- CH3OH + HI → CH3I + H2O
- CH3I + CO → CH3COI
- CH3COI + H2O → CH3COOH + HI
Two related processes exist for the carbonylation of methanol: the rhodium-catalyzed Monsanto process, and the iridium-catalyzed Cativa process. The latter process is greener and more efficient and has largely supplanted the former process.[21] Catalytic amounts of water are used in both processes, but the Cativa process requires less, so the water-gas shift reaction is suppressed, and fewer by-products are formed.
By altering the process conditions, acetic anhydride may also be produced in plants using rhodium catalysis.[40]
Acetaldehyde oxidation
Prior to the commercialization of the Monsanto process, most acetic acid was produced by oxidation of acetaldehyde. This remains the second-most-important manufacturing method, although it is usually not competitive with the carbonylation of methanol. The acetaldehyde can be produced by hydration of acetylene. This was the dominant technology in the early 1900s.[41]
Zdroj:https://en.wikipedia.org?pojem=Acetic_acid
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk