A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| Part of a series of articles about |
| Quantum mechanics |
|---|

The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position and momentum, can be simultaneously known. In other words, the more accurately one property is measured, the less accurately the other property can be known.
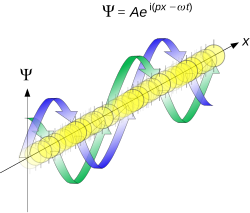
More formally, the uncertainty principle is any of a variety of mathematical inequalities asserting a fundamental limit to the product of the accuracy of certain related pairs of measurements on a quantum system, such as position, x, and momentum, p.[1] Such paired-variables are known as complementary variables or canonically conjugate variables.
First introduced in 1927 by German physicist Werner Heisenberg,[2][3][4][5] the formal inequality relating the standard deviation of position σx and the standard deviation of momentum σp was derived by Earle Hesse Kennard[6] later that year and by Hermann Weyl[7] in 1928:
where is the reduced Planck constant.
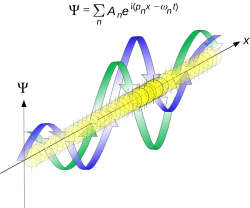
The quintessentially quantum mechanical uncertainty principle comes in many forms other than position–momentum. The energy–time relationship is widely used to relate quantum state lifetime to measured energy widths but its formal derivation is fraught with confusing issues about the nature of time. The basic principle has been extended in numerous directions; it must be considered in many kinds of fundamental physical measurements.
Position-momentum
It is vital to illustrate how the principle applies to relatively intelligible physical situations since it is indiscernible on the macroscopic[8] scales that humans experience. Two alternative frameworks for quantum physics offer different explanations for the uncertainty principle. The wave mechanics picture of the uncertainty principle is more visually intuitive, but the more abstract matrix mechanics picture formulates it in a way that generalizes more easily.
Mathematically, in wave mechanics, the uncertainty relation between position and momentum arises because the expressions of the wavefunction in the two corresponding orthonormal bases in Hilbert space are Fourier transforms of one another (i.e., position and momentum are conjugate variables). A nonzero function and its Fourier transform cannot both be sharply localized at the same time.[9] A similar tradeoff between the variances of Fourier conjugates arises in all systems underlain by Fourier analysis, for example in sound waves: A pure tone is a sharp spike at a single frequency, while its Fourier transform gives the shape of the sound wave in the time domain, which is a completely delocalized sine wave. In quantum mechanics, the two key points are that the position of the particle takes the form of a matter wave, and momentum is its Fourier conjugate, assured by the de Broglie relation p = ħk, where k is the wavenumber.
In matrix mechanics, the mathematical formulation of quantum mechanics, any pair of non-commuting self-adjoint operators representing observables are subject to similar uncertainty limits. An eigenstate of an observable represents the state of the wavefunction for a certain measurement value (the eigenvalue). For example, if a measurement of an observable A is performed, then the system is in a particular eigenstate Ψ of that observable. However, the particular eigenstate of the observable A need not be an eigenstate of another observable B: If so, then it does not have a unique associated measurement for it, as the system is not in an eigenstate of that observable.[10]
Visualization
The uncertainty principle can be visualized using the position- and momentum-space wavefunctions for one spinless particle with mass in one dimension.
The more localized the position-space wavefunction, the more likely the particle is to be found with the position coordinates in that region, and correspondingly the momentum-space wavefunction is less localized so the possible momentum components the particle could have are more widespread. Conversely, the more localized the momentum-space wavefunction, the more likely the particle is to be found with those values of momentum components in that region, and correspondingly the less localized the position-space wavefunction, so the position coordinates the particle could occupy are more widespread. These wavefunctions are Fourier transforms of each other: mathematically, the uncertainty principle expresses the relationship between conjugate variables in the transform.
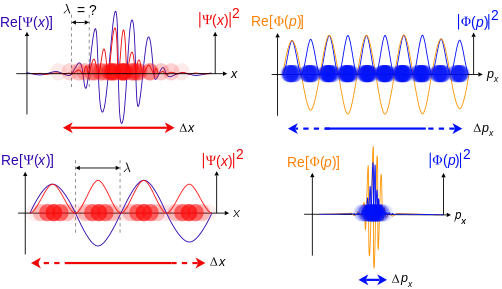
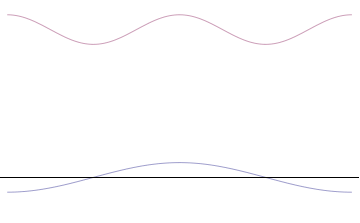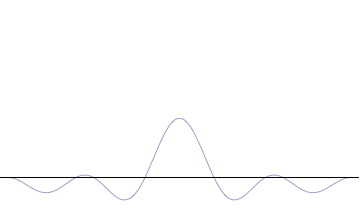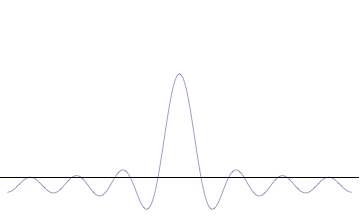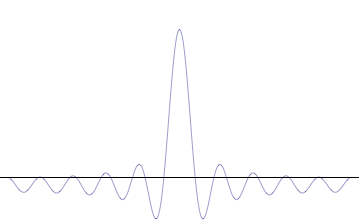
Top: If wavelength λ is unknown, so are momentum p, wave-vector k and energy E (de Broglie relations). As the particle is more localized in position space, Δx is smaller than for Δpx.
Bottom: If λ is known, so are p, k, and E. As the particle is more localized in momentum space, Δp is smaller than for Δx.
Wave mechanics interpretation
According to the de Broglie hypothesis, every object in the universe is associated with a wave. Thus every object, from an elementary particle to atoms, molecules and on up to planets and beyond are subject to the uncertainty principle.
The time-independent wave function of a single-moded plane wave of wavenumber k0 or momentum p0 is
The Born rule states that this should be interpreted as a probability density amplitude function in the sense that the probability of finding the particle between a and b is
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk