A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyridine[1] | |||
| Systematic IUPAC name
Azabenzene | |||
| Other names
Azine
Azinine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.464 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H5N | |||
| Molar mass | 79.102 g·mol−1 | ||
| Appearance | Colorless liquid[2] | ||
| Odor | Nauseating, fish-like[3] | ||
| Density | 0.9819 g/mL (20 °C)[4] | ||
| Melting point | −41.63 °C (−42.93 °F; 231.52 K)[4] | ||
| Boiling point | 115.2 °C (239.4 °F; 388.3 K)[4] | ||
| Miscible[4] | |||
| log P | 0.65[5] | ||
| Vapor pressure | 16 mmHg (20 °C)[3] | ||
| Acidity (pKa) | 5.23 (pyridinium)[6] | ||
| Conjugate acid | Pyridinium | ||
| −48.7·10−6 cm3/mol[7] | |||
| Thermal conductivity | 0.166 W/(m·K)[8] | ||
Refractive index (nD)
|
1.5095 (20 °C)[4] | ||
| Viscosity | 0.879 cP (25 °C)[9] | ||
| 2.215 D[10] | |||
| Thermochemistry[11] | |||
Heat capacity (C)
|
132.7 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
100.2 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.782 MJ/mol | ||
| Hazards[15] | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Low to moderate hazard[13] | ||
| GHS labelling: | |||
  [12] [12]
| |||
| Danger | |||
| H225, H302, H312, H315, H319, H332[12] | |||
| P210, P280, P301+P312, P303+P361+P353, P304+P340+P312, P305+P351+P338[12] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 20 °C (68 °F; 293 K)[16] | ||
| 482 °C (900 °F; 755 K)[16] | |||
| Explosive limits | 1.8–12.4%[3] | ||
Threshold limit value (TLV)
|
5 ppm (TWA) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
891 mg/kg (rat, oral) 1500 mg/kg (mouse, oral) 1580 mg/kg (rat, oral)[14] | ||
LC50 (median concentration)
|
9000 ppm (rat, 1 hr)[14] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (15 mg/m3)[3] | ||
REL (Recommended)
|
TWA 5 ppm (15 mg/m3)[3] | ||
IDLH (Immediate danger)
|
1000 ppm[3] | ||
| Related compounds | |||
Related amines
|
Picoline Quinoline | ||
Related compounds
|
Aniline Pyrimidine Piperidine | ||
| Supplementary data page | |||
| Pyridine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
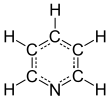
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom (=N−). It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow. due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity.[page needed][17] The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.[2]
Properties

Physical properties
Pyridine is diamagnetic. Its critical parameters are: pressure 5.63 MPa, temperature 619 K and volume 248 cm3·mol−1.[19] In the temperature range 340–426 °C its vapor pressure p can be described with the Antoine equation
where T is temperature, A = 4.16272, B = 1371.358 K and C = −58.496 K.[20]
Zdroj:https://en.wikipedia.org?pojem=PyridineText je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk