
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium manganate(VII)
| |
| Systematic IUPAC name
Potassium permanganate | |
| Other names
Chameleon mineral
Condy's crystals Permanganate of potash Hypermangan Purple potion powder | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.874 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1490 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| KMnO4 | |
| Molar mass | 158.034 g/mol |
| Appearance | Purplish-bronze-gray needles purple in solution[1] |
| Odor | odorless |
| Density | 2.7 g/cm3[2]: 4.83 |
| Melting point | 240 °C (464 °F; 513 K) (decomposes) |
| 76 g/L (25 °C)[2] 250 g/L (65 °C) | |
| Solubility | decomposes in alcohol and organic solvents |
| +20.0·10−6 cm3/mol[2]: 4.134 | |
Refractive index (nD)
|
1.59 |
| Structure[3] | |
| Orthorhombic, oP24 | |
| Pnma, No. 62 | |
a = 0.909 nm, b = 0.572 nm, c = 0.741 nm
| |
Formula units (Z)
|
4 |
| Thermochemistry | |
Heat capacity (C)
|
119.2 J/mol K |
Std molar
entropy (S⦵298) |
171.7 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−813.4 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
-713.8 kJ/mol |
| Pharmacology | |
| D08AX06 (WHO) V03AB18 (WHO) | |
| Hazards | |
| GHS labelling: | |
  
| |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1090 mg/kg (oral, rat)[4] |
| Related compounds | |
Other anions
|
Potassium pertechnetate Potassium perrhenate |
Other cations
|
Sodium permanganate Ammonium permanganate Calcium permanganate Silver permanganate |
Related manganates
|
Potassium hypomanganate Potassium manganate |
Related compounds
|
Manganese heptoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
| Clinical data | |
|---|---|
| License data | |
| Identifiers | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.874 |
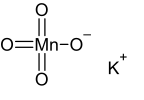
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and MnO−
4, an intensely pink to purple solution.
Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines.[5] In 2000, worldwide production was estimated at 30,000 tons.[5]
Properties
Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2− ligands are bound to a manganese(VII) center.[citation needed]
Structure
KMnO4 forms orthorhombic crystals with constants: a = 910.5 pm, b = 572.0 pm, c = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions.[6] In the solid (as in solution), each MnO−4 centre is tetrahedral. The Mn–O distances are 1.62 Å.[7]
Color
The purplish-black color of solid potassium permanganate, and the intensely pink to purple color of its solutions, is caused by its permanganate anion, which gets its color from a strong charge-transfer absorption band caused by excitation of electrons from oxo ligand orbitals to empty orbitals of the manganese(VII) center.[8]
Medical uses
Mechanism of action
Potassium permanganate functions as a strong oxidising agent.[9] Through this mechanism it results in disinfection, astringent effects, and decreased smell.[9]
Clinical use
Potassium permanganate is used for a number of skin conditions.[10] This includes fungal infections of the foot, impetigo, pemphigus, superficial wounds, dermatitis, and topical ulcers.[11][10] For topical ulcers it is used together with procaine benzylpenicillin.[10] Typically it is used in skin conditions that produce a lot of liquid.[11] It can be applied as a soaked dressing or a bath.
It can be used in children and adults.[12] It can be applied as a soaked dressing or a bath.[13] Petroleum jelly may be used on the nails before soaking to prevent their discoloration.[14] For treating eczema, it is recommended using for a few days at a time due to the possibility of it irritating the skin.[15]
The U.S. Food and Drug Administration does not recommend its use in the crystal or tablet form. It should only be used in a diluted liquid form.[16]
Historical use
Potassium permanganate was first made in the 1600s and came into common medical use at least as early as the 1800s.[17] During World War I Canadian soldiers were given potassium permanganate (to be applied mixed with an ointment) in an effort to prevent sexually transmitted infections (resulting mostly in violet stained genitals.)[18] Some have attempted to bring about an abortion by putting it in the vagina, though this is not effective.[19][20][21] Other historical uses have included an effort to wash out the stomach in those with strychnine or picrotoxin poisoning.[22]
Side effects
Side effects from topical use may include irritation of the skin and discoloration of clothing.[23] A harsh burn on a child from an undissolved tablet has been reported.[24] Higher concentration solutions can result in chemical burns.[25] Therefore, the British National Formulary recommends 100 mg be dissolved in a liter of water before use to form a 1:10,000 (0.01%) solution.[26] [27][28] Wrapping the dressings soaked with potassium permanganate is not recommended.[citation needed]
Potassium permanganate is toxic if taken by mouth.[29] Side effects may include nausea, vomiting, and shortness of breath may occur.[30] If a sufficiently large amount (about 10 grams) is eaten death may occur.[30]
Concentrated solutions when drunk have resulted in acute respiratory distress syndrome or swelling of the airway.[31] Recommended measures for those who have ingested potassium permanganate include gastroscopy.[31] Activated charcoal or medications to cause vomiting are not recommended. While medications like ranitidine and N-acetylcysteine may be used in toxicity, evidence for this use is poor.[31]
Pharmaceuticals
In the United States the FDA requires tablets of the medication to be sold by prescription.[32] Potassium permanganate, however, does not have FDA approved uses and therefore non medical grade potassium permanganate is sometimes used for medical purposes.
It is available under a number of brand names including Permasol, Koi Med Tricho-Ex, and Kalii permanganas RFF.[33] It is occasionally called "Condy's crystals".[34]
Veterinary medicine
Potassium permanganate may be used to prevent the spread of glanders among horses.[35]
Industrial and other uses
Almost all applications of potassium permanganate exploit its oxidizing properties.[36] As a strong oxidant that does not generate toxic byproducts, KMnO4 has many niche uses.[citation needed]
Water treatment
Potassium permanganate is used extensively in the water treatment industry. It is used as a regeneration chemical to remove iron and hydrogen sulfide (rotten egg smell) from well water via a "manganese greensand" filter. "Pot-Perm" is also obtainable at pool supply stores and is used additionally to treat wastewater. Historically it was used to disinfect drinking water[37][38] and can turn the water pink.[39] Modern hiking and survivalist guides advise against using potassium permanganate in the field because it is difficult to dose correctly.[40] It currently finds application in the control of nuisance organisms such as zebra mussels in fresh water collection and treatment systems.[41]
Synthesis of organic compounds

A major application of KMnO4 is as a reagent for the synthesis of organic compounds.[42] Significant amounts are required for the synthesis of ascorbic acid, chloramphenicol, saccharin, isonicotinic acid, and pyrazinoic acid.[36]
KMnO4 is used in qualitative organic analysis to test for the presence of unsaturation. It is sometimes referred to as Baeyer's reagent after the German organic chemist Adolf von Baeyer. The reagent is an alkaline solution of potassium permanganate. Reaction with double or triple bonds (-C=C- or -C≡C-) causes the color to fade from purplish-pink to brown. Aldehydes and formic acid (and formates) also give a positive test.[43] The test is antiquated.

KMnO4 solution is a common thin layer chromatography (TLC) stain for the detection of oxidizable functional groups, such as alcohols, aldehydes, alkenes, and ketones. Such compounds result in a white to orange spot on TLC plates.[44][45][46]
Analytical use
Potassium permanganate can be used to quantitatively determine the total oxidizable organic material in an aqueous sample. The value determined is known as the permanganate value. In analytical chemistry, a standardized aqueous solution of KMnO4 is sometimes used as an oxidizing titrant for redox titrations (permanganometry). As potassium permanganate is titrated, the solution becomes a light shade of purple, which darkens as excess of the titrant is added to the solution. In a related way, it is used as a reagent to determine the Kappa number of wood pulp. For the standardization of KMnO4 solutions, reduction by oxalic acid is often used.[47] In agricultural chemistry, it is used for estimation of active carbon in soil.[48]
Aqueous, acidic solutions of KMnO4 are used to collect gaseous mercury in flue gas during stationary source emissions testing.[49]
In histology, potassium permanganate was used as a bleaching agent.[50][51]
Fruit preservation
Ethylene absorbents extend storage time of bananas even at high temperatures. This effect can be exploited by packing bananas in polyethylene together with potassium permanganate. By removing ethylene by oxidation, the permanganate delays the ripening, increasing the fruit's shelf life up to 4 weeks without the need for refrigeration.[52][53][54]
Survival kits
Potassium permanganate is sometimes included in survival kits: as a hypergolic fire starter (when mixed with glycerol antifreeze from a car radiator);[55][56][57] as a water sterilizer; and for creating distress signals on snow.[58]
Fire service
Potassium permanganate is added to "plastic sphere dispensers" to create backfires, burnouts, and controlled burns. Polymer spheres resembling ping-pong balls containing small amounts of permanganate are injected with ethylene glycol and projected towards the area where ignition is desired, where they spontaneously ignite seconds later.[59][60] Both handheld[60] helicopter-[59] unmanned aircraft systems (UAS) or boat-mounted[60] plastic sphere dispensers are used.
Other uses
Potassium permanganate is one of the principal chemicals used in the film and television industries to "age" props and set dressings. Its ready conversion to brown MnO2 creates "hundred-year-old" or "ancient" looks on hessian cloth (burlap), ropes, timber and glass.[61]
Potassium permanganate can be used to oxidize cocaine paste to purify it and increase its stability. This led to the Drug Enforcement Administration launching Operation Purple in 2000, with the goal of monitoring the world supply of potassium permanganate; however, potassium permanganate derivatives and substitutes were soon used thereafter to avoid the operation.[62]
Potassium permanganate is used as an oxidizing agent in the synthesis of cocaine and methcathinone.[63]
Potassium permanganate is one of a number of possible treatments for Ichthyophthirius multifiliis (commonly known as "ich"), a parasite that infects and usually kills freshwater aquarium fish.
History
In 1659, Johann Rudolf Glauber fused a mixture of the mineral pyrolusite (manganese dioxide, MnO2) and potassium carbonate to obtain a material that, when dissolved in water, gave a green solution (potassium manganate) which slowly shifted to violet and then finally red.[64] The reaction that produced the color changes that Glauber observed in his solution of potassium permanganate and potassium manganate (K2MnO4) is now known as the "chemical chameleon". This report represents the first description of the production of potassium permanganate.[65] Just under 200 years later, London chemist Henry Bollmann Condy had an interest in disinfectants; he found that fusing pyrolusite with sodium hydroxide (NaOH) and dissolving it in water produced a solution with disinfectant properties. He patented this solution, and marketed it as 'Condy's Fluid'. Although effective, the solution was not very stable. This was overcome by using potassium hydroxide (KOH) rather than NaOH. This was more stable, and had the advantage of easy conversion to the equally effective potassium permanganate crystals. This crystalline material was known as 'Condy's crystals' or 'Condy's powder'. Potassium permanganate was comparatively easy to manufacture, so Condy was subsequently forced to spend considerable time in litigation to stop competitors from marketing similar products.[66]
Early photographers used it as a component of flash powder. It is now replaced with other oxidizers, due to the instability of permanganate mixtures.[citation needed]
Preparationedit
Potassium permanganate is produced industrially from manganese dioxide, which also occurs as the mineral pyrolusite. In 2000, worldwide production was estimated at 30,000 tonnes.[36] The MnO2 is fused with potassium hydroxide and heated in air or with another source of oxygen, like potassium nitrate or potassium chlorate.[36] This process gives potassium manganate:
(With sodium hydroxide, the end product is not sodium manganate but an Mn(V) compound, which is one reason why the potassium permanganate is more commonly used than sodium permanganate. Furthermore, the potassium salt crystallizes better.[36])
The potassium manganate is then converted into permanganate by electrolytic oxidation in alkaline media:
Other methodsedit
Zdroj:https://en.wikipedia.org?pojem=Potassium_permanganateText je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk



