
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

In quantum mechanics, an atomic orbital (/ˈɔːrbɪtəl/) is a function describing the location and wave-like behavior of an electron in an atom.[1] This function describes the electron's charge distribution around the atom's nucleus, and can be used to calculate the probability of finding an electron in a specific region around the nucleus.[2]
Each orbital in an atom is characterized by a set of values of the three quantum numbers n, ℓ, and ml, which respectively correspond to the electron's energy, its orbital angular momentum, and its orbital angular momentum projected along a chosen axis (magnetic quantum number). The orbitals with a well-defined magnetic quantum number are generally complex-valued. Real-valued orbitals can be formed as linear combinations of ml and -ml orbitals, and are often labeled using the associated harmonic polynomials (e.g., xy, x2 − y2) which describe their angular structure.
An orbital can be occupied by a maximum of two electrons, each with its own projection of spin . The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically (g, h, i, k, ...),[3] omitting j[4][5] because some languages do not distinguish between the letters "i" and "j".[6]
Atomic orbitals are the basic building blocks of the atomic orbital model (or electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of an atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d, and f orbitals, respectively, though for higher values of quantum number n, particularly when the atom bears a positive charge, the energies of certain sub-shells become very similar and so the order in which they are said to be populated by electrons (e.g., Cr = 4s13d5 and Cr2+ = 3d4) can be rationalized only somewhat arbitrarily.

Electron properties
With the development of quantum mechanics and experimental findings (such as the two slit diffraction of electrons), it was found that the electrons orbiting a nucleus could not be fully described as particles, but needed to be explained by wave–particle duality. In this sense, electrons have the following properties:
Wave-like properties:
- Electrons do not orbit a nucleus in the manner of a planet orbiting a star, but instead exist as standing waves. Thus the lowest possible energy an electron can take is similar to the fundamental frequency of a wave on a string. Higher energy states are similar to harmonics of that fundamental frequency.
- The electrons are never in a single point location, though the probability of interacting with the electron at a single point can be found from the electron's wave function. The electron's charge acts like it is smeared out in space in a continuous distribution, proportional at any point to the squared magnitude of the electron's wave function.
Particle-like properties:
- The number of electrons orbiting a nucleus can be only an integer.
- Electrons jump between orbitals like particles. For example, if one photon strikes the electrons, only one electron changes state as a result.
- Electrons retain particle-like properties such as: each wave state has the same electric charge as its electron particle. Each wave state has a single discrete spin (spin up or spin down) depending on its superposition.
Thus, electrons cannot be described simply as solid particles. An analogy might be that of a large and often oddly shaped "atmosphere" (the electron), distributed around a relatively tiny planet (the nucleus). Atomic orbitals exactly describe the shape of this "atmosphere" only when one electron is present. When more electrons are added, the additional electrons tend to more evenly fill in a volume of space around the nucleus so that the resulting collection ("electron cloud"[7]) tends toward a generally spherical zone of probability describing the electron's location, because of the uncertainty principle.
Formal quantum mechanical definition
Atomic orbitals may be defined more precisely in formal quantum mechanical language. They are approximate solutions to the Schrödinger equation for the electrons bound to the atom by the electric field of the atom's nucleus. Specifically, in quantum mechanics, the state of an atom, i.e., an eigenstate of the atomic Hamiltonian, is approximated by an expansion (see configuration interaction expansion and basis set) into linear combinations of anti-symmetrized products (Slater determinants) of one-electron functions. The spatial components of these one-electron functions are called atomic orbitals. (When one considers also their spin component, one speaks of atomic spin orbitals.) A state is actually a function of the coordinates of all the electrons, so that their motion is correlated, but this is often approximated by this independent-particle model of products of single electron wave functions.[8] (The London dispersion force, for example, depends on the correlations of the motion of the electrons.)
In atomic physics, the atomic spectral lines correspond to transitions (quantum leaps) between quantum states of an atom. These states are labeled by a set of quantum numbers summarized in the term symbol and usually associated with particular electron configurations, i.e., by occupation schemes of atomic orbitals (for example, 1s2 2s2 2p6 for the ground state of neon-term symbol: 1S0).
This notation means that the corresponding Slater determinants have a clear higher weight in the configuration interaction expansion. The atomic orbital concept is therefore a key concept for visualizing the excitation process associated with a given transition. For example, one can say for a given transition that it corresponds to the excitation of an electron from an occupied orbital to a given unoccupied orbital. Nevertheless, one has to keep in mind that electrons are fermions ruled by the Pauli exclusion principle and cannot be distinguished from each other.[9] Moreover, it sometimes happens that the configuration interaction expansion converges very slowly and that one cannot speak about simple one-determinant wave function at all. This is the case when electron correlation is large.
Fundamentally, an atomic orbital is a one-electron wave function, even though many electrons are not in one-electron atoms, and so the one-electron view is an approximation. When thinking about orbitals, we are often given an orbital visualization heavily influenced by the Hartree–Fock approximation, which is one way to reduce the complexities of molecular orbital theory.
Types of orbital
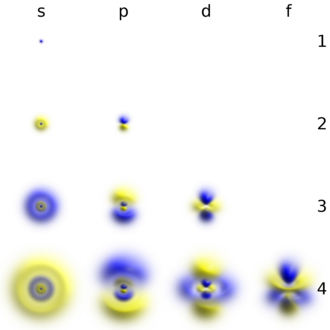
Atomic orbitals can be the hydrogen-like "orbitals" which are exact solutions to the Schrödinger equation for a hydrogen-like "atom" (i.e., atom with one electron). Alternatively, atomic orbitals refer to functions that depend on the coordinates of one electron (i.e., orbitals) but are used as starting points for approximating wave functions that depend on the simultaneous coordinates of all the electrons in an atom or molecule. The coordinate systems chosen for orbitals are usually spherical coordinates (r, θ, φ) in atoms and Cartesian (x, y, z) in polyatomic molecules. The advantage of spherical coordinates here is that an orbital wave function is a product of three factors each dependent on a single coordinate: ψ(r, θ, φ) = R(r) Θ(θ) Φ(φ). The angular factors of atomic orbitals Θ(θ) Φ(φ) generate s, p, d, etc. functions as real combinations of spherical harmonics Yℓm(θ, φ) (where ℓ and m are quantum numbers). There are typically three mathematical forms for the radial functions R(r) which can be chosen as a starting point for the calculation of the properties of atoms and molecules with many electrons:
- The hydrogen-like orbitals are derived from the exact solutions of the Schrödinger equation for one electron and a nucleus, for a hydrogen-like atom. The part of the function that depends on distance r from the nucleus has radial nodes and decays as .
- The Slater-type orbital (STO) is a form without radial nodes but decays from the nucleus as does a hydrogen-like orbital.
- The form of the Gaussian type orbital (Gaussians) has no radial nodes and decays as .
Although hydrogen-like orbitals are still used as pedagogical tools, the advent of computers has made STOs preferable for atoms and diatomic molecules since combinations of STOs can replace the nodes in hydrogen-like orbitals. Gaussians are typically used in molecules with three or more atoms. Although not as accurate by themselves as STOs, combinations of many Gaussians can attain the accuracy of hydrogen-like orbitals.
History
The term "orbital" was coined by Robert S. Mulliken in 1932 as short for one-electron orbital wave function.[10] Niels Bohr explained around 1913 that electrons might revolve around a compact nucleus with definite angular momentum.[11] Bohr's model was an improvement on the 1911 explanations of Ernest Rutherford, that of the electron moving around a nucleus. Japanese physicist Hantaro Nagaoka published an orbit-based hypothesis for electron behavior as early as 1904.[12] These theories were each built upon new observations starting with simple understanding and becoming more correct and complex. Explaining the behavior of these electron "orbits" was one of the driving forces behind the development of quantum mechanics.[13]
Early models
With J. J. Thomson's discovery of the electron in 1897,[14] it became clear that atoms were not the smallest building blocks of nature, but were rather composite particles. The newly discovered structure within atoms tempted many to imagine how the atom's constituent parts might interact with each other. Thomson theorized that multiple electrons revolve in orbit-like rings within a positively charged jelly-like substance,[15] and between the electron's discovery and 1909, this "plum pudding model" was the most widely accepted explanation of atomic structure.
Shortly after Thomson's discovery, Hantaro Nagaoka predicted a different model for electronic structure.[12] Unlike the plum pudding model, the positive charge in Nagaoka's "Saturnian Model" was concentrated into a central core, pulling the electrons into circular orbits reminiscent of Saturn's rings. Few people took notice of Nagaoka's work at the time,[16] and Nagaoka himself recognized a fundamental defect in the theory even at its conception, namely that a classical charged object cannot sustain orbital motion because it is accelerating and therefore loses energy due to electromagnetic radiation.[17] Nevertheless, the Saturnian model turned out to have more in common with modern theory than any of its contemporaries.
Bohr atom
In 1909, Ernest Rutherford discovered that the bulk of the atomic mass was tightly condensed into a nucleus, which was also found to be positively charged. It became clear from his analysis in 1911 that the plum pudding model could not explain atomic structure. In 1913, Rutherford's post-doctoral student, Niels Bohr, proposed a new model of the atom, wherein electrons orbited the nucleus with classical periods, but were permitted to have only discrete values of angular momentum, quantized in units ħ.[11] This constraint automatically allowed only certain electron energies. The Bohr model of the atom fixed the problem of energy loss from radiation from a ground state (by declaring that there was no state below this), and more importantly explained the origin of spectral lines.

After Bohr's use of Einstein's explanation of the photoelectric effect to relate energy levels in atoms with the wavelength of emitted light, the connection between the structure of electrons in atoms and the emission and absorption spectra of atoms became an increasingly useful tool in the understanding of electrons in atoms. The most prominent feature of emission and absorption spectra (known experimentally since the middle of the 19th century), was that these atomic spectra contained discrete lines. The significance of the Bohr model was that it related the lines in emission and absorption spectra to the energy differences between the orbits that electrons could take around an atom. This was, however, not achieved by Bohr through giving the electrons some kind of wave-like properties, since the idea that electrons could behave as matter waves was not suggested until eleven years later. Still, the Bohr model's use of quantized angular momenta and therefore quantized energy levels was a significant step toward the understanding of electrons in atoms, and also a significant step towards the development of quantum mechanics in suggesting that quantized restraints must account for all discontinuous energy levels and spectra in atoms.
With de Broglie's suggestion of the existence of electron matter waves in 1924, and for a short time before the full 1926 Schrödinger equation treatment of hydrogen-like atoms, a Bohr electron "wavelength" could be seen to be a function of its momentum; so a Bohr orbiting electron was seen to orbit in a circle at a multiple of its half-wavelength. The Bohr model for a short time could be seen as a classical model with an additional constraint provided by the 'wavelength' argument. However, this period was immediately superseded by the full three-dimensional wave mechanics of 1926. In our current understanding of physics, the Bohr model is called a semi-classical model because of its quantization of angular momentum, not primarily because of its relationship with electron wavelength, which appeared in hindsight a dozen years after the Bohr model was proposed.
The Bohr model was able to explain the emission and absorption spectra of hydrogen. The energies of electrons in the n = 1, 2, 3, etc. states in the Bohr model match those of current physics. However, this did not explain similarities between different atoms, as expressed by the periodic table, such as the fact that helium (two electrons), neon (10 electrons), and argon (18 electrons) exhibit similar chemical inertness. Modern quantum mechanics explains this in terms of electron shells and subshells which can each hold a number of electrons determined by the Pauli exclusion principle. Thus the n = 1 state can hold one or two electrons, while the n = 2 state can hold up to eight electrons in 2s and 2p subshells. In helium, all n = 1 states are fully occupied; the same is true for n = 1 and n = 2 in neon. In argon, the 3s and 3p subshells are similarly fully occupied by eight electrons; quantum mechanics also allows a 3d subshell but this is at higher energy than the 3s and 3p in argon (contrary to the situation for hydrogen) and remains empty.
Modern conceptions and connections to the Heisenberg uncertainty principle
Immediately after Heisenberg discovered his uncertainty principle,[18] Bohr noted that the existence of any sort of wave packet implies uncertainty in the wave frequency and wavelength, since a spread of frequencies is needed to create the packet itself.[19] In quantum mechanics, where all particle momenta are associated with waves, it is the formation of such a wave packet which localizes the wave, and thus the particle, in space. In states where a quantum mechanical particle is bound, it must be localized as a wave packet, and the existence of the packet and its minimum size implies a spread and minimal value in particle wavelength, and thus also momentum and energy. In quantum mechanics, as a particle is localized to a smaller region in space, the associated compressed wave packet requires a larger and larger range of momenta, and thus larger kinetic energy. Thus the binding energy to contain or trap a particle in a smaller region of space increases without bound as the region of space grows smaller. Particles cannot be restricted to a geometric point in space, since this would require infinite particle momentum.
In chemistry, Erwin Schrödinger, Linus Pauling, Mulliken and others noted that the consequence of Heisenberg's relation was that the electron, as a wave packet, could not be considered to have an exact location in its orbital. Max Born suggested that the electron's position needed to be described by a probability distribution which was connected with finding the electron at some point in the wave-function which described its associated wave packet. The new quantum mechanics did not give exact results, but only the probabilities for the occurrence of a variety of possible such results. Heisenberg held that the path of a moving particle has no meaning if we cannot observe it, as we cannot with electrons in an atom.
In the quantum picture of Heisenberg, Schrödinger and others, the Bohr atom number n for each orbital became known as an n-sphere[citation needed] in a three-dimensional atom and was pictured as the most probable energy of the probability cloud of the electron's wave packet which surrounded the atom.
Orbital names
Orbital notation and subshells
Orbitals have been given names, which are usually given in the form:
where X is the energy level corresponding to the principal quantum number n; type is a lower-case letter denoting the shape or subshell of the orbital, corresponding to the angular momentum quantum number ℓ.
For example, the orbital 1s (pronounced as the individual numbers and letters: "'one' 'ess'") is the lowest energy level (n = 1) and has an angular quantum number of ℓ = 0, denoted as s. Orbitals with ℓ = 1, 2 and 3 are denoted as p, d and f respectively.
The set of orbitals for a given n and ℓ is called a subshell, denoted
- .
The superscript y shows the number of electrons in the subshell. For example, the notation 2p4 indicates that the 2p subshell of an atom contains 4 electrons. This subshell has 3 orbitals, each with n = 2 and ℓ = 1.
X-ray notation
There is also another, less common system still used in X-ray science known as X-ray notation, which is a continuation of the notations used before orbital theory was well understood. In this system, the principal quantum number is given a letter associated with it. For n = 1, 2, 3, 4, 5, ..., the letters associated with those numbers are K, L, M, N, O, ... respectively.
Hydrogen-like orbitals
The simplest atomic orbitals are those that are calculated for systems with a single electron, such as the hydrogen atom. An atom of any other element ionized down to a single electron is very similar to hydrogen, and the orbitals take the same form. In the Schrödinger equation for this system of one negative and one positive particle, the atomic orbitals are the eigenstates of the Hamiltonian operator for the energy. They can be obtained analytically, meaning that the resulting orbitals are products of a polynomial series, and exponential and trigonometric functions. (see hydrogen atom).
For atoms with two or more electrons, the governing equations can be solved only with the use of methods of iterative approximation. Orbitals of multi-electron atoms are qualitatively similar to those of hydrogen, and in the simplest models, they are taken to have the same form. For more rigorous and precise analysis, numerical approximations must be used.
A given (hydrogen-like) atomic orbital is identified by unique values of three quantum numbers: n, ℓ, and mℓ. The rules restricting the values of the quantum numbers, and their energies (see below), explain the electron configuration of the atoms and the periodic table.
The stationary states (quantum states) of the hydrogen-like atoms are its atomic orbitals.[clarification needed] However, in general, an electron's behavior is not fully described by a single orbital. Electron states are best represented by time-depending "mixtures" (linear combinations) of multiple orbitals. See Linear combination of atomic orbitals molecular orbital method.
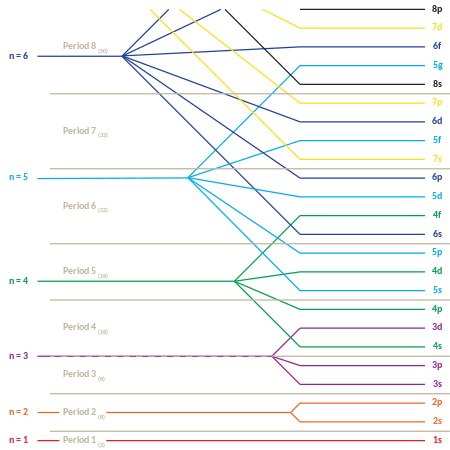
The quantum number n first appeared in the Bohr model where it determines the radius of each circular electron orbit. In modern quantum mechanics however, n determines the mean distance of the electron from the nucleus; all electrons with the same value of n lie at the same average distance. For this reason, orbitals with the same value of n are said to comprise a "shell". Orbitals with the same value of n and also the same value of ℓ are even more closely related, and are said to comprise a "subshell".
Quantum numbers
Because of the quantum mechanical nature of the electrons around a nucleus, atomic orbitals can be uniquely defined by a set of integers known as quantum numbers. These quantum numbers occur only in certain combinations of values, and their physical interpretation changes depending on whether real or complex versions of the atomic orbitals are employed.
Complex orbitals
In physics, the most common orbital descriptions are based on the solutions to the hydrogen atom, where orbitals are given by the product between a radial function and a pure spherical harmonic. The quantum numbers, together with the rules governing their possible values, are as follows:
The principal quantum number n describes the energy of the electron and is always a positive integer. In fact, it can be any positive integer, but for reasons discussed below, large numbers are seldom encountered. Each atom has, in general, many orbitals associated with each value of n; these orbitals together are sometimes called electron shells.
The azimuthal quantum number ℓ describes the orbital angular momentum of each electron and is a non-negative integer. Within a shell where n is some integer n0, ℓ ranges across all (integer) values satisfying the relation . For instance, the n = 1 shell has only orbitals with , and the n = 2 shell has only orbitals with , and . The set of orbitals associated with a particular value of ℓ are sometimes collectively called a subshell.
The magnetic quantum number, , describes the projection of the orbital angular momentum along a chosen axis. It determines the magnitude of the current circulating around that axis and the orbital contribution to the magnetic moment of an electron via the Ampèrian loop model.[20] Within a subshell , obtains the integer values in the range .
The above results may be summarized in the following table. Each cell represents a subshell, and lists the values of available in that subshell. Empty cells represent subshells that do not exist.
| ℓ = 0 (s) | ℓ = 1 (p) | ℓ = 2 (d) | ℓ = 3 (f) | ℓ = 4 (g) | ... | |
|---|---|---|---|---|---|---|
| n = 1 | ... | |||||
| n = 2 | 0 | −1, 0, 1 | ... | |||
| n = 3 | 0 | −1, 0, 1 | −2, −1, 0, 1, 2 | ... | ||
| n = 4 | 0 | −1, 0, 1 | −2, −1, 0, 1, 2 | −3, −2, −1, 0, 1, 2, 3 | ... | |
| n = 5 | 0 | −1, 0, 1 | −2, −1, 0, 1, 2 | −3, −2, −1, 0, 1, 2, 3 | −4, −3, −2, −1, 0, 1, 2, 3, 4 | ... |
| ... | ... | ... | ... | ... | ... | ... |
Subshells are usually identified by their - and -values. is represented by its numerical value, but is represented by a letter as follows: 0 is represented by 's', 1 by 'p', 2 by 'd', 3 by 'f', and 4 by 'g'. For instance, one may speak of the subshell with and as a '2s subshell'.
Each electron also has a spin quantum number, s, which describes the spin of each electron (spin up or spin down). The number s can be +1/2 or −1/2.
The Pauli exclusion principle states that no two electrons in an atom can have the same values of all four quantum numbers. If there are two electrons in an orbital with given values for three quantum numbers, (n, ℓ, m), these two electrons must differ in their spin.
The above conventions imply a preferred axis (for example, the z direction in Cartesian coordinates), and they also imply a preferred direction along this preferred axis. Otherwise there would be no sense in distinguishing m = +1 from m = −1. As such, the model is most useful when applied to physical systems that share these symmetries. The Stern–Gerlach experiment—where an atom is exposed to a magnetic field—provides one such example.[21]
Real orbitals
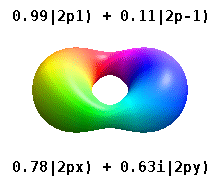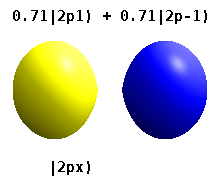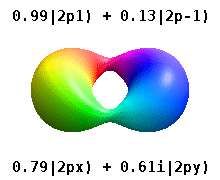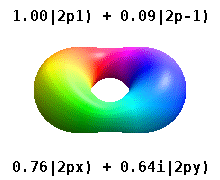
Instead of the complex orbitals described above, it is common, especially in the chemistry literature, to use real atomic orbitals. These real orbitals arise from simple linear combinations of complex orbitals. Using the Condon–Shortley phase convention, real orbitals are related to complex orbitals in the same way that the real spherical harmonics are related to complex spherical harmonics. Letting denote a complex orbital with quantum numbers , , and , the real orbitals may be defined by[22]
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk




















