
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| Corals Temporal range:
| |
|---|---|

| |
| A coral outcrop on the Great Barrier Reef, Australia | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Cnidaria |
| Subphylum: | Anthozoa Ehrenberg, 1834 |
| Subdivisions | |
Corals are colonial marine invertebrates within the class Anthozoa of the phylum Cnidaria. They typically form compact colonies of many identical individual polyps. Coral species include the important reef builders that inhabit tropical oceans and secrete calcium carbonate to form a hard skeleton.
A coral "group" is a colony of very many genetically identical polyps. Each polyp is a sac-like animal typically only a few millimeters in diameter and a few centimeters in height. A set of tentacles surround a central mouth opening. Each polyp excretes an exoskeleton near the base. Over many generations, the colony thus creates a skeleton characteristic of the species which can measure up to several meters in size. Individual colonies grow by asexual reproduction of polyps. Corals also breed sexually by spawning: polyps of the same species release gametes simultaneously overnight, often around a full moon. Fertilized eggs form planulae, a mobile early form of the coral polyp which, when mature, settles to form a new colony.
Although some corals are able to catch plankton and small fish using stinging cells on their tentacles, most corals obtain the majority of their energy and nutrients from photosynthetic unicellular dinoflagellates of the genus Symbiodinium that live within their tissues. These are commonly known as zooxanthellae and give the coral color. Such corals require sunlight and grow in clear, shallow water, typically at depths less than 60 metres (200 feet; 33 fathoms), but corals in the genus Leptoseris have been found as deep as 172 metres (564 feet; 94 fathoms).[1] Corals are major contributors to the physical structure of the coral reefs that develop in tropical and subtropical waters, such as the Great Barrier Reef off the coast of Australia. These corals are increasingly at risk of bleaching events where polyps expel the zooxanthellae in response to stress such as high water temperature or toxins.
Other corals do not rely on zooxanthellae and can live globally in much deeper water, such as the cold-water genus Lophelia which can survive as deep as 3,300 metres (10,800 feet; 1,800 fathoms).[2] Some have been found as far north as the Darwin Mounds, northwest of Cape Wrath, Scotland, and others off the coast of Washington state and the Aleutian Islands.
Taxonomy
The classification of corals has been discussed for millennia, owing to having similarities to both plants and animals. Aristotle's pupil Theophrastus described the red coral, korallion, in his book on stones, implying it was a mineral, but he described it as a deep-sea plant in his Enquiries on Plants, where he also mentions large stony plants that reveal bright flowers when under water in the Gulf of Heroes.[3] Pliny the Elder stated boldly that several sea creatures including sea nettles and sponges "are neither animals nor plants, but are possessed of a third nature (tertia natura)".[4] Petrus Gyllius copied Pliny, introducing the term zoophyta for this third group in his 1535 book On the French and Latin Names of the Fishes of the Marseilles Region; it is popularly but wrongly supposed that Aristotle created the term.[4] Gyllius further noted, following Aristotle, how hard it was to define what was a plant and what was an animal.[4] The Babylonian Talmud refers to coral among a list of types of trees, and the 11th-century French commentator Rashi describes it as "a type of tree (מין עץ) that grows underwater that goes by the (French) name "coral."[5]
The Persian polymath Al-Biruni (d.1048) classified sponges and corals as animals, arguing that they respond to touch.[6] Nevertheless, people believed corals to be plants until the eighteenth century when William Herschel used a microscope to establish that coral had the characteristic thin cell membranes of an animal.[7]
Presently, corals are classified as species of animals within the sub-classes Hexacorallia and Octocorallia of the class Anthozoa in the phylum Cnidaria.[8] Hexacorallia includes the stony corals and these groups have polyps that generally have a 6-fold symmetry. Octocorallia includes blue coral and soft corals and species of Octocorallia have polyps with an eightfold symmetry, each polyp having eight tentacles and eight mesenteries. The group of corals is paraphyletic because the sea anemones are also in the sub-class Hexacorallia.
Systematics
This section's use of external links may not follow Wikipedia's policies or guidelines. (August 2023) |
The delineation of coral species is challenging as hypotheses based on morphological traits contradict hypotheses formed via molecular tree-based processes.[9] As of 2020, there are 2175 identified separate coral species, 237 of which are currently endangered,[10] making distinguishing corals to be the utmost of importance in efforts to curb extinction.[9] Adaptation and delineation continues to occur in species of coral[11] in order to combat the dangers posed by the climate crisis. Corals are colonial modular organisms formed by asexually produced and genetically identical modules called polyps. Polyps are connected by living tissue to produce the full organism.[12] The living tissue allows for inter module communication (interaction between each polyp),[12] which appears in colony morphologies produced by corals, and is one of the main identifying characteristics for a species of coral.[12]
There are two main classifications for corals: hard coral (scleractinian and stony coral)[13] which form reefs by a calcium carbonate base, with polyps that bear six stiff tentacles,[14] and soft coral (Alcyonacea and ahermatypic coral)[13] which are pliable and formed by a colony of polyps with eight feather-like tentacles.[14] These two classifications arose from differentiation in gene expressions in their branch tips[12] and bases that arose through developmental signaling pathways such as Hox, Hedgehog, Wnt, BMP etc.
Scientists typically select Acropora as research models since they are the most diverse genus of hard coral, having over 120 species.[12] Most species within this genus have polyps which are dimorphic:[15] axial polyps grow rapidly and have lighter coloration, while radial polyps are small and are darker in coloration.[12][16] In the Acropora genus, gamete synthesis and photosynthesis occur at the basal[17] polyps, growth occurs mainly at the radial polyps. Growth at the site of the radial polyps encompasses two processes: asexual reproduction via mitotic cell proliferation,[12] and skeleton deposition of the calcium carbonate via extra cellular matrix (EMC) proteins acting as differentially expressed (DE) signaling genes[12] between both branch tips and bases. These processes lead to colony differentiation, which is the most accurate distinguisher between coral species.[9] In the Acropora genus, colony differentiation through up-regulation and down-regulation of DEs.[12]
Systematic studies of soft coral species have faced challenges due to a lack of taxonomic knowledge.[9] Researchers have not found enough variability within the genus to confidently delineate similar species, due to a low rate in mutation of mitochondrial DNA.[18]
Environmental factors, such as the rise of temperatures and acid levels in our oceans account for some speciation of corals in the form of species lost.[12] Various coral species have heat shock proteins (HSP) that are also in the category of DE across species.[12] These HSPs help corals combat the increased temperatures they are facing which lead to protein denaturing, growth loss, and eventually coral death.[12] Approximately 33% of coral species are on the International Union for Conservation of Nature's endangered species list and at risk of species loss.[19] Ocean acidification (falling pH levels in the oceans) is threatening the continued species growth and differentiation of corals.[12] Mutation rates of Vibrio shilonii, the reef pathogen responsible for coral bleaching, heavily outweigh the typical reproduction rates of coral colonies when pH levels fall.[20] Thus, corals are unable to mutate their HSPs and other climate change preventative genes to combat the increase in temperature and decrease in pH at a competitive rate to these pathogens responsible for coral bleaching,[20] resulting in species loss.
Anatomy
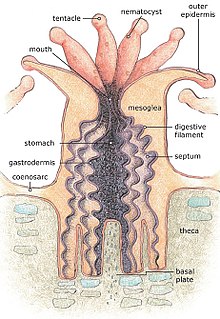
For most of their life corals are sessile animals of colonies of genetically identical polyps. Each polyp varies from millimeters to centimeters in diameter, and colonies can be formed from many millions of individual polyps. Stony coral, also known as hard coral, polyps produce a skeleton composed of calcium carbonate to strengthen and protect the organism. This is deposited by the polyps and by the coenosarc, the living tissue that connects them. The polyps sit in cup-shaped depressions in the skeleton known as corallites. Colonies of stony coral are markedly variable in appearance; a single species may adopt an encrusting, plate-like, bushy, columnar or massive solid structure, the various forms often being linked to different types of habitat, with variations in light level and water movement being significant.[21]
The body of the polyp may be roughly compared in a structure to a sac, the wall of which is composed of two layers of cells. The outer layer is known technically as the ectoderm, the inner layer as the endoderm. Between ectoderm and endoderm is a supporting layer of gelatinous substance termed mesoglea, secreted by the cell layers of the body wall.[22] The mesoglea can contain skeletal elements derived from cells migrated from the ectoderm.
The sac-like body built up in this way is attached to a hard surface, which in hard corals are cup-shaped depressions in the skeleton known as corallites. At the center of the upper end of the sac lies the only opening called the mouth, surrounded by a circle of tentacles which resemble glove fingers. The tentacles are organs which serve both for tactile sense and for the capture of food.[22] Polyps extend their tentacles, particularly at night, often containing coiled stinging cells (cnidocytes) which pierce, poison and firmly hold living prey paralyzing or killing them. Polyp prey includes plankton such as copepods and fish larvae. Longitudinal muscular fibers formed from the cells of the ectoderm allow tentacles to contract to convey the food to the mouth. Similarly, circularly disposed muscular fibres formed from the endoderm permit tentacles to be protracted or thrust out once they are contracted.[22] In both stony and soft corals, the polyps can be retracted by contracting muscle fibres, with stony corals relying on their hard skeleton and cnidocytes for defense. Soft corals generally secrete terpenoid toxins to ward off predators.[21]
In most corals, the tentacles are retracted by day and spread out at night to catch plankton and other small organisms. Shallow-water species of both stony and soft corals can be zooxanthellate, the corals supplementing their plankton diet with the products of photosynthesis produced by these symbionts.[21] The polyps interconnect by a complex and well-developed system of gastrovascular canals, allowing significant sharing of nutrients and symbionts.[23]
The external form of the polyp varies greatly. The column may be long and slender, or may be so short in the axial direction that the body becomes disk-like. The tentacles may number many hundreds or may be very few, in rare cases only one or two. They may be simple and unbranched, or feathery in pattern. The mouth may be level with the surface of the peristome, or may be projecting and trumpet-shaped.[22]
Soft corals
Soft corals have no solid exoskeleton as such. However, their tissues are often reinforced by small supportive elements known as sclerites made of calcium carbonate. The polyps of soft corals have eight-fold symmetry, which is reflected in the Octo in Octocorallia.[24]
Soft corals vary considerably in form, and most are colonial. A few soft corals are stolonate, but the polyps of most are connected by sheets of tissue called coenosarc, and in some species these sheets are thick and the polyps deeply embedded in them. Some soft corals encrust other sea objects or form lobes. Others are tree-like or whip-like and have a central axial skeleton embedded at their base in the matrix of the supporting branch.[25] These branches are composed of a fibrous protein called gorgonin or of a calcified material.
Stony corals

The polyps of stony corals have six-fold symmetry. In stony corals, the tentacles are cylindrical and taper to a point, but in soft corals they are pinnate with side branches known as pinnules. In some tropical species, these are reduced to mere stubs and in some, they are fused to give a paddle-like appearance.[26]
Coral skeletons are biocomposites (mineral + organics) of calcium carbonate, in the form of calcite or aragonite. In scleractinian corals, "centers of calcification" and fibers are clearly distinct structures differing with respect to both morphology and chemical compositions of the crystalline units.[27][28] The organic matrices extracted from diverse species are acidic, and comprise proteins, sulphated sugars and lipids; they are species specific.[29] The soluble organic matrices of the skeletons allow to differentiate zooxanthellae and non-zooxanthellae specimens.[30]
Ecology
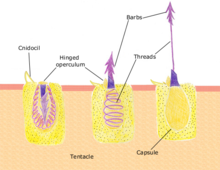
Feeding
Polyps feed on a variety of small organisms, from microscopic zooplankton to small fish. The polyp's tentacles immobilize or kill prey using stinging cells called nematocysts. These cells carry venom which they rapidly release in response to contact with another organism. A dormant nematocyst discharges in response to nearby prey touching the trigger (Cnidocil). A flap (operculum) opens and its stinging apparatus fires the barb into the prey. The venom is injected through the hollow filament to immobilise the prey; the tentacles then manoeuvre the prey into the stomach. Once the prey is digested the stomach reopens allowing the elimination of waste products and the beginning of the next hunting cycle.[31]: 24
Intracellular symbionts
Many corals, as well as other cnidarian groups such as sea anemones form a symbiotic relationship with a class of dinoflagellate algae, zooxanthellae of the genus Symbiodinium, which can form as much as 30% of the tissue of a polyp.[31]: 23–24 Typically, each polyp harbors one species of alga, and coral species show a preference for Symbiodinium.[32] Young corals are not born with zooxanthellae, but acquire the algae from the surrounding environment, including the water column and local sediment.[33] The main benefit of the zooxanthellae is their ability to photosynthesize which supplies corals with the products of photosynthesis, including glucose, glycerol, also amino acids, which the corals can use for energy.[34] Zooxanthellae also benefit corals by aiding in calcification, for the coral skeleton, and waste removal.[35][36] In addition to the soft tissue, microbiomes are also found in the coral's mucus and (in stony corals) the skeleton, with the latter showing the greatest microbial richness.[37]
The zooxanthellae benefit from a safe place to live and consume the polyp's carbon dioxide, phosphate and nitrogenous waste. Stressed corals will eject their zooxanthellae, a process that is becoming increasingly common due to strain placed on coral by rising ocean temperatures. Mass ejections are known as coral bleaching because the algae contribute to coral coloration; some colors, however, are due to host coral pigments, such as green fluorescent proteins (GFPs). Ejection increases the polyp's chance of surviving short-term stress and if the stress subsides they can regain algae, possibly of a different species, at a later time. If the stressful conditions persist, the polyp eventually dies.[38] Zooxanthellae are located within the coral cytoplasm and due to the algae's photosynthetic activity the internal pH of the coral can be raised; this behavior indicates that the zooxanthellae are responsible to some extent for the metabolism of their host corals.[39] Stony Coral Tissue Loss Disease has been associated with the breakdown of host-zooxanthellae physiology.[40] Moreover, Vibrio bacterium are known to have virulence traits used for host coral tissue damage and photoinhibition of algal symbionts.[41] Therefore, both coral and their symbiotic microorganisms could have evolved to harbour traits resistant to disease and transmission.
Reproduction
Corals can be both gonochoristic (unisexual) and hermaphroditic, each of which can reproduce sexually and asexually. Reproduction also allows coral to settle in new areas. Reproduction is coordinated by chemical communication.[clarify]
Sexual

Corals predominantly reproduce sexually. About 25% of hermatypic corals (reef-building stony corals) form single-sex (gonochoristic) colonies, while the rest are hermaphroditic.[citation needed] It is estimated more than 67% of coral are simultaneous hermaphrodites.[42]
Broadcasters
| External videos | |
|---|---|
 | |
About 75% of all hermatypic corals "broadcast spawn"[citation needed] by releasing gametes—eggs and sperm—into the water where they meet and fertilize to spread offspring. Corals often synchronize their time of spawning. This reproductive synchrony is essential so that male and female gametes can meet. Spawning frequently takes place in the evening or at night, and can occur as infrequently as once a year, and within a window of 10–30 minutes.[43][44] Synchronous spawning is very typical on the coral reef, and often, all corals spawn on the same night even when multiple species are present.[45] Synchronous spawning may form hybrids and is perhaps involved in coral speciation.[46]

Environmental cues that influence the release of gametes into the water vary from species to species. The cues involve temperature change, lunar cycle, day length, and possibly chemical signalling.[45] Other factors that affect the rhythmicity of organisms in marine habitats include salinity, mechanical forces, and pressure or magnetic field changes.[44]
Mass coral spawning often occurs at night on days following a full moon.[43][47] A full moon is equivalent to four to six hours of continuous dim light exposure, which can cause light-dependent reactions in protein.[43][44] Corals contain light-sensitive cryptochromes, proteins whose light-absorbing flavin structures are sensitive to different types of light. This allows corals such as Dipsastraea speciosa to detect and respond to changes in sunlight and moonlight.[43][44][48]
Moonlight itself may actually suppress coral spawning. The most immediate cue to cause spawning appears to be the dark portion of the night between sunset and moonrise. Over the lunar cycle, moonrise shifts progressively later, occurring after sunset on the day of the full moon. The resulting dark period between day-light and night-light removes the suppressive effect of moonlight and enables coral to spawn.[43][47]
The spawning event can be visually dramatic, clouding the usually clear water with gametes. Once released, gametes fertilize at the water's surface and form a microscopic larva called a planula, typically pink and elliptical in shape. A typical coral colony needs to release several thousand larvae per year to overcome the odds against formation of a new colony.[49][50]
Studies suggest that light pollution desynchronizes spawning in some coral species. In areas such as the Red Sea, as many as 10 out of 50 species may be showing spawning asynchrony, compared to 30 years ago. The establishment of new corals in the area has decreased and in some cases ceased. The area was previously considered a refuge for corals because mass bleaching events due to climate change had not been observed there.[43][51] Coral restoration techniques for coral reef management are being developed to increase fertilization rates, larval development, and settlement of new corals.[52]
Brooders
Brooding species are most often ahermatypic (not reef-building) in areas of high current or wave action. Brooders release only sperm, which is negatively buoyant, sinking onto the waiting egg carriers that harbor unfertilized eggs for weeks. Synchronous spawning events sometimes occur even with these species.[45] After fertilization, the corals release planula that are ready to settle.[35]

Planulae
The time from spawning to larval settlement is usually two to three days but can occur immediately or up to two months.[53] Broadcast-spawned planula larvae develop at the water's surface before descending to seek a hard surface on the benthos to which they can attach and begin a new colony.[54] The larvae often need a biological cue to induce settlement such as specific crustose coralline algae species or microbial biofilms.[55][56] High failure rates afflict many stages of this process, and even though thousands of eggs are released by each colony, few new colonies form. During settlement, larvae are inhibited by physical barriers such as sediment,[57] as well as chemical (allelopathic) barriers.[58] The larvae metamorphose into a single polyp and eventually develops into a juvenile and then adult by asexual budding and growth.
Asexual

Within a coral head, the genetically identical polyps reproduce asexually, either by budding (gemmation) or by dividing, whether longitudinally or transversely.
Budding involves splitting a smaller polyp from an adult.[49] As the new polyp grows, it forms its body parts. The distance between the new and adult polyps grows, and with it, the coenosarc (the common body of the colony). Budding can be intratentacular, from its oral discs, producing same-sized polyps within the ring of tentacles, or extratentacular, from its base, producing a smaller polyp.
Zdroj:https://en.wikipedia.org?pojem=Coral_microbiome
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk
