
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |

| |
| Names | |
|---|---|
| IUPAC name
2-Hydroxyethyl(trimethyl)azanium[1]
| |
| Preferred IUPAC name
2-Hydroxy-N,N,N-trimethylethan-1-aminium | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1736748 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.487 |
| EC Number |
|
| 324597 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
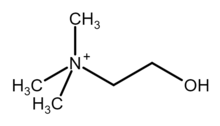
| [(CH3)3NCH2CH2OH+ | |
| Molar mass | 104.173 g·mol−1 |
| Appearance | Viscous colorless deliquescent liquid (choline hydroxide)[2] |
| Very soluble (choline hydroxide)[2] | |
| Solubility | soluble in ethanol,[2] insoluble in diethylether and chloroform[3] (choline hydroxide) |
| Structure | |
| Tetrahedral at the nitrogen atom | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3–6 g/kg (rat, oral)[2] |
| Safety data sheet (SDS) | 4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Choline (/ˈkoʊliːn/ KOH-leen)[4] is an essential nutrient for humans and many other animals, which was formerly classified as a B vitamin (vitamin B4).[5][6] It is a structural part of phospholipids and a methyl donor in metabolic one-carbon chemistry. The compound is related to trimethylglycine in the latter respect. It is a cation with the chemical formula [(CH3)3NCH2CH2OH+. Choline forms various salts, for example choline chloride and choline bitartrate.
Chemistry
Choline is a quaternary ammonium cation. The cholines are a family of water-soluble quaternary ammonium compounds.[6][7] Choline is the parent compound of the cholines class, consisting of ethanolamine residue having three methyl groups attached to the same nitrogen atom.[1] Choline hydroxide is known as choline base. It is hygroscopic and thus often encountered as a colorless viscous hydrated syrup that smells of trimethylamine (TMA). Aqueous solutions of choline are stable, but the compound slowly breaks down to ethylene glycol, polyethylene glycols, and TMA.[2]
Choline chloride can be made by treating TMA with 2-chloroethanol:[2]
- (CH3)3N + ClCH2CH2OH → [(CH3)3NCH2CH2OH+Cl−
The 2-chloroethanol can be generated from ethylene oxide.[how?] Choline has historically been produced from natural sources, such as via hydrolysis of lecithin.[2]
Choline as a nutrient
Choline is widespread in nature in living beings. In most animals, choline phospholipids are necessary components in cell membranes, in the membranes of cell organelles, and in very low-density lipoproteins.[5]
Choline is an essential nutrient for humans and many other animals.[5][6] Humans are capable of some de novo synthesis of choline but require additional choline in the diet to maintain health. Dietary requirements can be met by choline by itself or in the form of choline phospholipids, such as phosphatidylcholine.[5] Choline is not formally classified as a vitamin despite being an essential nutrient with an amino acid–like structure and metabolism.[3]
Choline is required to produce acetylcholine – a neurotransmitter – and S-adenosylmethionine (SAM), a universal methyl donor. Upon methylation SAM is transformed into S-adenosyl homocysteine.[5]
Symptomatic choline deficiency causes non-alcoholic fatty liver disease and muscle damage.[5] Excessive consumption of choline (greater than 7.5 grams per day) can cause low blood pressure, sweating, diarrhea and fish-like body smell due to trimethylamine, which forms in the metabolism of choline.[5][8] Rich dietary sources of choline and choline phospholipids include organ meats, egg yolks, dairy products, peanuts, certain beans, nuts and seeds. Vegetables with pasta and rice also contribute to choline intake in the American diet.[5][9]
Metabolism
Biosynthesis
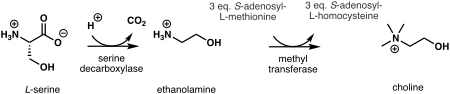
In plants, the first step in de novo biosynthesis of choline is the decarboxylation of serine into ethanolamine, which is catalyzed by a serine decarboxylase.[10] The synthesis of choline from ethanolamine may take place in three parallel pathways, where three consecutive N-methylation steps catalyzed by a methyl transferase are carried out on either the free-base,[11] phospho-bases,[12] or phosphatidyl-bases.[13] The source of the methyl group is S-adenosyl-L-methionine and S-adenosyl-L-homocysteine is generated as a side product.[14]

In humans and most other animals, de novo synthesis of choline is via the phosphatidylethanolamine N-methyltransferase (PEMT) pathway,[8] but biosynthesis is not enough to meet human requirements.[15] In the hepatic PEMT route, 3-phosphoglycerate (3PG) receives 2 acyl groups from acyl-CoA forming a phosphatidic acid. It reacts with cytidine triphosphate to form cytidine diphosphate-diacylglycerol. Its hydroxyl group reacts with serine to form phosphatidylserine which decarboxylates to ethanolamine and phosphatidylethanolamine (PE) forms. A PEMT enzyme moves three methyl groups from three S-adenosyl methionines (SAM) donors to the ethanolamine group of the phosphatidylethanolamine to form choline in the form of a phosphatidylcholine. Three S-adenosylhomocysteines (SAHs) are formed as a byproduct.[8]
Choline can also be released from more complex choline containing molecules. For example, phosphatidylcholines (PC) can be hydrolyzed to choline (Chol) in most cell types. Choline can also be produced by the CDP-choline route, cytosolic choline kinases (CK) phosphorylate choline with ATP to phosphocholine (PChol).[3] This happens in some cell types like liver and kidney. Choline-phosphate cytidylyltransferases (CPCT) transform PChol to CDP-choline (CDP-Chol) with cytidine triphosphate (CTP). CDP-choline and diglyceride are transformed to PC by diacylglycerol cholinephosphotransferase (CPT).[8]
In humans, certain PEMT-enzyme mutations and estrogen deficiency (often due to menopause) increase the dietary need for choline. In rodents, 70% of phosphatidylcholines are formed via the PEMT route and only 30% via the CDP-choline route.[8] In knockout mice, PEMT inactivation makes them completely dependent on dietary choline.[3]
Absorption
In humans, choline is absorbed from the intestines via the SLC44A1 (CTL1) membrane protein via facilitated diffusion governed by the choline concentration gradient and the electrical potential across the enterocyte membranes. SLC44A1 has limited ability to transport choline: at high concentrations part of it is left unabsorbed. Absorbed choline leaves the enterocytes via the portal vein, passes the liver and enters systemic circulation. Gut microbes degrade the unabsorbed choline to trimethylamine, which is oxidized in the liver to trimethylamine N-oxide.[8]
Phosphocholine and glycerophosphocholines are hydrolyzed via phospholipases to choline, which enters the portal vein. Due to their water solubility, some of them escape unchanged to the portal vein. Fat-soluble choline-containing compounds (phosphatidylcholines and sphingomyelins) are either hydrolyzed by phospholipases or enter the lymph incorporated into chylomicrons.[8]
Transport
In humans, choline is transported as a free molecule in blood. Choline–containing phospholipids and other substances, like glycerophosphocholines, are transported in blood lipoproteins. Blood plasma choline levels in healthy fasting adults is 7–20 micromoles per liter (μmol/L) and 10 μmol/L on average. Levels are regulated, but choline intake and deficiency alters these levels. Levels are elevated for about 3 hours after choline consumption. Phosphatidylcholine levels in the plasma of fasting adults is 1.5–2.5 mmol/L. Its consumption elevates the free choline levels for about 8–12 hours, but does not affect phosphatidylcholine levels significantly.[8]
Choline is a water-soluble ion and thus requires transporters to pass through fat-soluble cell membranes. Three types of choline transporters are known:[16]
- SLC5A7
- CTLs: CTL1 (SLC44A1), CTL2 (SLC44A2) and CTL4 (SLC44A4)
- OCTs: OCT1 (SLC22A1) and OCT2 (SLC22A2)
SLC5A7s are sodium- (Na+) and ATP-dependent transporters.[16][8] They have high binding affinity for choline, transport it primarily to neurons and are indirectly associated with the acetylcholine production.[8] Their deficient function causes hereditary weakness in the pulmonary and other muscles in humans via acetylcholine deficiency. In knockout mice, their dysfunction results easily in death with cyanosis and paralysis.[17]
CTL1s have moderate affinity for choline and transport it in almost all tissues, including the intestines, liver, kidneys, placenta and mitochondria. CTL1s supply choline for phosphatidylcholine and trimethylglycine production.[8] CTL2s occur especially in the mitochondria in the tongue, kidneys, muscles and heart. They are associated with the mitochondrial oxidation of choline to trimethylglycine. CTL1s and CTL2s are not associated with the acetylcholine production, but transport choline together via the blood–brain barrier. Only CTL2s occur on the brain side of the barrier. They also remove excess choline from the neurons back to blood. CTL1s occur only on the blood side of the barrier, but also on the membranes of astrocytes and neurons.[16]
OCT1s and OCT2s are not associated with the acetylcholine production.[8] They transport choline with low affinity. OCT1s transport choline primarily in the liver and kidneys; OCT2s in kidneys and the brain.[16]
Storage
Choline is stored in the cell membranes and organelles as phospholipids, and inside cells as phosphatidylcholines and glycerophosphocholines.[8]
Excretion
Even at choline doses of 2–8 g, little choline is excreted into urine in humans. Excretion happens via transporters that occur within kidneys (see transport). Trimethylglycine is demethylated in the liver and kidneys to dimethylglycine (tetrahydrofolate receives one of the methyl groups). Methylglycine forms, is excreted into urine, or is demethylated to glycine.[8]
Function
Choline and its derivatives have many functions in humans and in other organisms. The most notable function is that choline serves as a synthetic precursor for other essential cell components and signalling molecules, such as phospholipids that form cell membranes, the neurotransmitter acetylcholine, and the osmoregulator trimethylglycine (betaine). Trimethylglycine in turn serves as a source of methyl groups by participating in the biosynthesis of S-adenosylmethionine.[18][19]
Phospholipid precursor
Choline is transformed to different phospholipids, like phosphatidylcholines and sphingomyelins. These are found in all cell membranes and the membranes of most cell organelles.[3] Phosphatidylcholines are structurally important part of the cell membranes. In humans 40–50% of their phospholipids are phosphatidylcholines.[8]
Choline phospholipids also form lipid rafts in the cell membranes along with cholesterol. The rafts are centers, for example for receptors and receptor signal transduction enzymes.[3]
Phosphatidylcholines are needed for the synthesis of VLDLs: 70–95% of their phospholipids are phosphatidylcholines in humans.[8]
Choline is also needed for the synthesis of pulmonary surfactant, which is a mixture consisting mostly of phosphatidylcholines. The surfactant is responsible for lung elasticity, that is for lung tissue's ability to contract and expand. For example, deficiency of phosphatidylcholines in the lung tissues has been linked to acute respiratory distress syndrome.[20]
Phosphatidylcholines are excreted into bile and work together with bile acid salts as surfactants in it, thus helping with the intestinal absorption of lipids.[3]
Acetylcholine synthesis
Choline is needed to produce acetylcholine. This is a neurotransmitter which plays a necessary role in muscle contraction, memory and neural development, for example.[8] Nonetheless, there is little acetylcholine in the human body relative to other forms of choline.[3] Neurons also store choline in the form of phospholipids to their cell membranes for the production of acetylcholine.[8]
Source of trimethylglycine
In humans, choline is oxidized irreversibly in liver mitochondria to glycine betaine aldehyde by choline oxidases. This is oxidized by mitochondrial or cytosolic betaine-aldehyde dehydrogenases to trimethylglycine.[8] Trimethylglycine is a necessary osmoregulator. It also works as a substrate for the BHMT-enzyme, which methylates homocysteine to methionine. This is a S-adenosylmethionine (SAM) precursor. SAM is a common reagent in biological methylation reactions. For example, it methylates guanidines of DNA and certain lysines of histones. Thus it is part of gene expression and epigenetic regulation. Choline deficiency thus leads to elevated homocysteine levels and decreased SAM levels in blood.[8]
Content in foods
Choline occurs in foods as a free molecule and in the form of phospholipids, especially as phosphatidylcholines. Choline is highest in organ meats and egg yolks though it is found to a lesser degree in non-organ meats, grains, vegetables, fruit and dairy products. Cooking oils and other food fats have about 5 mg/100 g of total choline.[8] In the United States, food labels express the amount of choline in a serving as a percentage of daily value (%DV) based on the adequate intake of 550 mg/day. 100% of the daily value means that a serving of food has 550 mg of choline.[21] "Total choline" is defined as the sum of free choline and choline-containing phospholipids, without accounting for mass fraction.[22][23][8]
Human breast milk is rich in choline. Exclusive breastfeeding corresponds to about 120 mg of choline per day for the baby. Increase in a mother's choline intake raises the choline content of breast milk and low intake decreases it.[8] Infant formulas may or may not contain enough choline. In the EU and the US, it is mandatory to add at least 7 mg of choline per 100 kilocalories (kcal) to every infant formula. In the EU, levels above 50 mg/100 kcal are not allowed.[8][24]
Trimethylglycine is a functional metabolite of choline. It substitutes for choline nutritionally, but only partially.[3] High amounts of trimethylglycine occur in wheat bran (1,339 mg/100 g), toasted wheat germ (1,240 mg/100 g) and spinach (600–645 mg/100 g), for example.[22]
| Meats | Vegetables | ||
|---|---|---|---|
| Bacon, cooked | 124.89 | Bean, snap | 13.46 |
| Beef, trim-cut, cooked | 78.15 | Beetroot | 6.01 |
| Beef liver, pan fried | 418.22 | Broccoli | 40.06 |
| Chicken, roasted, with skin | 65.83 | Brussels sprout | 40.61 |
| Chicken, roasted, no skin | 78.74 | Cabbage | 15.45 |
| Chicken liver | 290.03 | Carrot | 8.79 |
| Cod, atlantic | 83.63 | Cauliflower | 39.10 |
| Ground beef, 75–85% lean, broiled | 79.32–82.35 | Sweetcorn, yellow | 21.95 |
| Pork loin cooked | 102.76 | Cucumber | 5.95 |
| Shrimp, canned | 70.60 | Lettuce, iceberg | 6.70 |
| Dairy products (cow) | Lettuce, romaine | 9.92 | |
| Butter, salted | 18.77 | Pea | 27.51 |
| Cheese | 16.50–27.21 | Sauerkraut | 10.39 |
| Cottage cheese | 18.42 | Spinach | 22.08 |
| Milk, whole/skimmed | 14.29–16.40 | Sweet potato | 13.11 |
| Sour cream | 20.33 | Tomato | 6.74 |
| Yogurt, plain | 15.20 | Zucchini | 9.36 |
| Grains | Fruits | ||
| Oat bran, raw | 58.57 | Apple | 3.44 |
| Oats, plain | 7.42 | Avocado | 14.18 |
| Rice, white | 2.08 | Banana
Zdroj:https://en.wikipedia.org?pojem=Choline Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Analytika
Antropológia Aplikované vedy Bibliometria Dejiny vedy Encyklopédie Filozofia vedy Forenzné vedy Humanitné vedy Knižničná veda Kryogenika Kryptológia Kulturológia Literárna veda Medzidisciplinárne oblasti Metódy kvantitatívnej analýzy Metavedy Metodika Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok. www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk | |

