
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| Bladder cancer | |
|---|---|
 | |
| Transitional cell carcinoma of the bladder. The white in the bladder is contrast. | |
| Specialty | Oncology, urology |
| Symptoms | Blood in the urine, pain with urination[1] |
| Usual onset | 65 to 84 years old[2] |
| Types | Transitional cell carcinoma, squamous cell carcinoma, adenocarcinoma[1] |
| Risk factors | Smoking, family history, prior radiation therapy, frequent bladder infections, certain chemicals[1] |
| Diagnostic method | Cystoscopy with tissue biopsies[1] |
| Treatment | Surgery, radiation therapy, chemotherapy, immunotherapy[1] |
| Prognosis | Five-year survival rates ~77% (US)[2] |
| Frequency | 549,000 new cases (2018)[3] |
| Deaths | 200,000 (2018)[3] |
Bladder cancer is any of several types of cancer arising from the tissues of the urinary bladder.[1] Symptoms include blood in the urine, pain with urination, and low back pain.[1] It is caused when epithelial cells that line the bladder become malignant.[4]
Risk factors for bladder cancer include smoking, family history, prior radiation therapy, frequent bladder infections, and exposure to certain chemicals.[1] The most common type is transitional cell carcinoma.[1] Other types include squamous cell carcinoma and adenocarcinoma.[1] Diagnosis is typically by cystoscopy with tissue biopsies.[5] Staging of the cancer is determined by transurethral resection and medical imaging.[1][6][7]
Treatment depends on the stage of the cancer.[1] It may include some combination of surgery, radiation therapy, chemotherapy, or immunotherapy.[1] Surgical options may include transurethral resection, partial or complete removal of the bladder, or urinary diversion.[1] The typical five-year survival rates in the United States is 77%, Canada is 75%, and Europe is 68%.[2][8][9]
Bladder cancer, as of 2018, affected about 1.6 million people globally with 549,000 new cases and 200,000 deaths.[3] Age of onset is most often between 65 and 84 years of age.[2] Males are more often affected than females.[2] In 2018, the highest rate of bladder cancer occurred in Southern and Western Europe followed by North America with rates of 15, 13, and 12 cases per 100,000 people.[3] The highest rates of bladder cancer deaths were seen in Northern Africa and Western Asia followed by Southern Europe.[3]
Signs and symptoms
Bladder cancer characteristically causes blood in the urine, which may be visible or detectable only by microscope. Blood in the urine is the most common symptom in bladder cancer, and is painless. Visible blood in the urine may be of only short duration, and a urine test may be required to confirm non-visible blood. Between 80 and 90% of people with bladder cancer initially presented with visible blood.[10] Blood in the urine may also be caused by other conditions, such as bladder or ureteric stones, infection, kidney disease, kidney cancers or vascular malformations, though these conditions (except kidney cancers) would typically be painful.[citation needed]
Other possible symptoms include pain during urination, frequent urination, or feeling the need to urinate without being able to do so. These signs and symptoms are not specific to bladder cancer, and may also be caused by non-cancerous conditions, including prostate infections, overactive bladder or cystitis. Some rare forms of bladder cancer like urachal adenocarcinoma produce mucin, which is then excreted in the urine causing it to be thick.[11]
People with advanced disease may have pelvic or bony pain, lower-extremity swelling, or flank pain.[12] Rarely, a palpable mass can be detected on physical examination.[13]
Causes
Smoking
Tobacco smoking is the main known contributor to urinary bladder cancer; in most populations, smoking is associated with over half of bladder cancer cases in men and one-third of cases among women,[14] however these proportions have reduced over recent years since there are fewer smokers in Europe and North America.[15] There is an almost linear relationship between smoking duration (in years), pack years and bladder cancer risk. A risk plateau at smoking about 15 cigarettes a day can be observed (meaning that those who smoke 15 cigarettes a day are approximately at the same risk as those smoking 30 cigarettes a day). Smoking in any form (cigar, cigarette, pipe, Egyptian waterpipe and smokeless tobacco) increases the risk for bladder cancer.[16] Quitting smoking reduces the risk. Risk of bladder cancer decreases by 30% within 1–4 years and continues to decrease by 60% at 25 years after smoking cessation.[17] However, former smokers will most likely always be at a higher risk of bladder cancer compared to people who have never smoked.[15] Passive smoking also appears to be a risk.[18][19]
Opium consumption increases the risk of bladder cancer threefold, while concurrent use of opium and tobacco increases the risk of bladder cancer fivefold when compared to the general population.[20]
Occupational exposure
Thirty percent of bladder tumors probably result from occupational exposure in the workplace to carcinogens. Occupational or circumstantial exposure to the following substances has been implicated as a cause of bladder cancer; benzidine (dyes manufacturing), 4-aminobiphenyl (rubber industry), 2-naphtylamine (azo dyes manufacturing, foundry fumes, rubber industry, cigarette smoke and cancer research), phenacetin (analgesic), arsenic and chlorinated aliphatic hydrocarbons in drinking water, auramine (dye manufacturing), magenta (dye manufacturing), ortho-toluidine (dye manufacturing), epoxy and polyurethane resin hardening agents (plastics industry), chlornaphazine, coal-tar pitch.[21][22][23][24][25] Occupations at risk are bus drivers, rubber workers, painters, motor mechanics, leather (including shoe) workers, blacksmiths, machine setters, and mechanics.[26][27] Hairdressers are thought to be at risk as well because of their frequent exposure to permanent hair dyes.[28]
Infection
Infection with Schistosoma haematobium (bilharzia or schistosomiasis) may cause bladder cancer, particularly of the squamous cell type.[29] Schistosoma eggs induces a chronic inflammatory state in the bladder wall resulting in tissue fibrosis.[30] Higher levels of N-nitroso compounds has been detected in urine samples of people with schistosomiasis.[31] N-Nitroso compounds have been implicated in the pathogenesis of schistosomiasis related bladder cancer. They cause alkylation DNA damage, specially Guanine to Adenine transition mutations in the HRAS and p53 tumor suppressor gene.[32] Mutations of p53 are detected in 73% of the tumors, BCL-2 mutations accounting for 32% and the combination of the two accounting for 13%.[33] Other causes of squamous cell carcinoma of the bladder include chronic catheterizations in people with a spinal cord injury and history of treatment with cyclophosphamide.[34][35]
Diet
The American Institute for Cancer Research have stated that there is strong evidence that drinking water containing arsenic increases the risk of bladder cancer.[36]
High consumption of animal fat and dietary cholesterol increases bladder cancer risk in men.[37]
Ingestion of aristolochic acid present in many Chinese herbal medications has been shown to cause urothelial carcinoma and kidney failure.[38] Aristolochic acid activates peroxidase in the urothelium and causes transversion mutation in the TP53 tumor suppressor gene.[citation needed]
Other
People who undergo external beam radiotherapy (EBRT) for prostate cancer have a higher risk of developing invasive bladder cancer.[39]
In addition to these major risk factors there are also numerous other modifiable factors that are less strongly (i.e. 10–20% risk increase) associated with bladder cancer, for example, obesity.[40] Although these could be considered as minor effects, risk reduction in the general population could still be achieved by reducing the prevalence of a number of smaller risk factor together.[41]
Genetics
Mutations in FGFR3, TP53, PIK3CA, KDM6A, ARID1A, KMT2D, HRAS, TERT, KRAS, CREBBP, RB1 and TSC1 genes may be associated with some cases of bladder cancer.[42][43][44] Deletions of parts or whole of chromosome 9 is common in bladder cancer.[45] Low grade cancer are known to harbor mutations in RAS pathway and the fibroblast growth factor receptor 3 (FGFR3) gene, both of which play a role in the MAPK/ERK pathway. p53 and RB gene mutations are implicated in high-grade muscle invasive tumors.[46] Eighty nine percent of muscle invasive cancers have mutations in chromatin remodeling and histone modifying genes.[47] Deletion of both copies of the GSTM1 gene has a modest increase in risk of bladder cancer. GSTM1 gene product glutathione S-transferase M1 (GSTM1) participates in the detoxification process of carcinogens such as polycyclic aromatic hydrocarbons found in cigarette smoke.[48] Similarly, mutations in NAT2 (N-acetyltransferase) is associated with increased risk for bladder cancer. N-acetyltransferase helps in detoxification of carcinogens like aromatic amines (also present in cigarette smoke).[49] Various single-nucleotide polymorphisms in PSCA gene present on chromosome 8 have shown to increase the risk for bladder cancer. PSCA gene promoter region has an androgen response region. Loss of reactivity of this region to androgens is hypothesized as a cause of more aggressive tumors in women (unlike in men who have higher amount of androgen).[50]
Muscle invasive bladder cancer are heterogeneous in nature. In general, they can be genetically classified into basal and luminal subtypes. Basal subtype show alterations involving RB and NFE2L2 and luminal type show changes in FGFR3 and KDM6A genes.[51] Basal subtype are subdivided into basal and claudin low-type group and are aggressive and show metastasis at presentation, however they respond to platinum based chemotherapy. Luminal subtype can be subdivided into p53-like and luminal. p53-like tumors of luminal subtype although not as aggressive as basal type, show resistance to chemotherapy[52]
Diagnosis


Currently, the best diagnosis of the state of the bladder is by way of cystoscopy, which is a procedure in which a flexible or rigid tube (called a cystoscope) bearing a camera and various instruments is inserted into the bladder through the urethra. The flexible procedure allows for a visual inspection of the bladder, for minor remedial work to be undertaken and for samples of suspicious lesions to be taken for a biopsy. A rigid cystoscope is used under general anesthesia in the operating room and can support remedial work and biopsies as well as more extensive tumor removal. Unlike papillary lesion, which grow into the bladder cavity and are readily visible, carcinoma in situ lesion are flat and obscure. Detection of carcinoma in situ lesions requires multiple biopsies from different areas of interior bladder wall.[53] Photodynamic detection (blue light cystoscopy) can aid in the detection of carcinoma in situ. In photodynamic detection, a dye is instilled into the bladder with the help of a catheter. Cancer cells take up this dye and are visible under blue light, providing visual clues on areas to be biopsied or resected.[54]
However, visual detection in any form listed above, is not sufficient for establishing pathological classification, cell type or the stage of the present tumor. A so-called cold cup biopsy during an ordinary cystoscopy (rigid or flexible) will not be sufficient for pathological staging either. Hence, a visual detection needs to be followed by transurethral surgery. The procedure is called transurethral resection of bladder tumor (TURBT). Further, a rectal and vaginal bimanual examination should be carried out before and after the TURBT to assess whether there is a palpable mass or if the tumour is fixed ("tethered") to the pelvic wall. The pathological classification and staging information obtained by the TURBT-procedure, is of fundamental importance for making the appropriate choice of ensuing treatment and/or follow-up routines.[55]
If invasive or high grade (includes carcinoma in situ) cancer is detected on TURBT, an MRI and/or CT scan of the abdomen and pelvis or urogram and CT chest should be conducted for disease staging and to look for cancer spread (metastasis).[56] Increase in alkaline phosphatase levels without evidence of liver disease should be evaluated for bone metastasis by a bone scan.[57] Although 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT has been explored as a viable method for staging, there is no consensus to support its role in routine clinical evaluations.[54]
Urine cytology can be obtained in voided urine or at the time of the cystoscopy ("bladder washing"). Cytology is not very sensitive for low-grade or grade 1 tumors (a negative result cannot reliably exclude bladder cancer) but has a high specificity (a positive result reliably detects bladder cancer).[58] There are newer non-invasive urine bound markers available as aids in the diagnosis of bladder cancer, including human complement factor H-related protein, high-molecular-weight carcinoembryonic antigen, and nuclear matrix protein 22 (NMP22).[59] In United States the FDA has approved NMP22, NMP22 BladderChek, and UroVysion tests for detection and surveillance of bladder cancer and ImmunoCyt, BTA-TRAK, and BTA-STAT tests have been approved for surveillance only. BTA-STAT and BladderChek can be performed in the clinic and others are done in the laboratory.[60][61] Other non-invasive urine based tests include the CertNDx Bladder Cancer Assay, which detects FGFR3 mutation and Urine Bladder Cancer test (UBC), which is a sandwich ELISA for Cytokeratin 8/18 fragment. Likewise, NMP22 is a sandwich ELISA and NMP22 BladderChek is a dipstick immunoassay, both of them detect nuclear mitotic apparatus protein (NuMA) tumor marker (a type of nuclear matrix protein).[62] UroVysion is a fluorescence in situ hybridization which detects aneuploidy in chromosomes 3, 7, 17 and loss of the 9p21 locus.[63][64] ImmunoCyt is an Immunofluorescence test which detects glycosylated CEA and MUCIN-like antigens (M344, LDQ10, 19A11).[62][63] BTA-STAT is a dipstick immunoassay for detection of human complement factor H-related protein. BTA-TRAK is a sandwich ELISA which also detects human complement factor H-related protein.[62] Sensitivities across biomarkers ranged from 0.57 to 0.82 and specificities from 0.74 to 0.88. Biomarkers fared better when used in combination with urine cytology than when used alone. However, detection accuracy is poor for low grade cancers and 10% cancers are still missed.[60] Current guidelines do not recommended using urinary biomarkers for detection and surveillance.[65]
Classification

| Type | Relative incidence | Subtypes |
|---|---|---|
| Transitional cell carcinoma | 95%[66][67] | Papillary (70%[66]) |
| Non-papillary (30%[66]) | ||
| Non-transitional cell carcinoma | 5% [66][67] | Squamous cell carcinomas, adenocarcinomas, sarcomas, small cell carcinomas, and secondary deposits from cancers elsewhere in the body.[67] |
Non-papillary carcinoma includes carcinoma in situ (CIS), microinvasive carcinoma and frankly invasive carcinoma.[68] Carcinoma in situ (CIS) invariably consists of cytologically high-grade tumour cells.[69]
Transitional cell carcinoma can undergo differentiation (25%) into its variants.[68][70][71] When seen under a microscope, papillary transitional cell carcinoma can present in its typical form or as one of its variations (squamous, glandular differentiation or micropapillary variant). Different variations of non-papillary transitional cell carcinoma are listed below.
| Variant | Histology | Percentage of non-papillary cases | Implications[72] |
|---|---|---|---|
| Squamous differentiation | Presence of intercellular bridges or keratinization | 60% | Outcomes similar to conventional transitional cell carcinoma |
| Glandular differentiation | Presence of true glandular spaces | 10% | |
| Sarcomatoid foci | Presence of both epithelial and mesenchymal differentiation | 7% | Clinically aggressive[73] |
| Micropapillary variant | Resembles papillary serous carcinoma of the ovary or resembling micropapillary carcinoma of breast or lung[74] | 3.7% | Clinically aggressive, early cystectomy recommended |
| Urothelial carcinoma with small tubules and microcystic form | Presence of cysts with a size range of microscopic to 1-2mm | Rare | |
| Lymphoepithelioma-like carcinoma | Resembles lymphoepithelioma of the nasopharynx | ||
| Lymphoma-like and plasmacytoid variants | Malignant cells resemble cells of malignant lymphoma or plasmacytoma | ||
| Nested variant | Histologically look similar to von Brunn's nests | Can be misdiagnosed as benign von Brunn's nests or non-invasive low-grade papillary urothelial carcinoma | |
| Urothelial carcinoma with giant cells | Presence of epithelial tumour giant cells and looks similar to giant cell carcinoma of the lung | ||
| Trophoblastic differentiation | Presence of syncytiotrophoblastic giant cells or choriocarcinomatous differentiation, may express HCG | ||
| Clear cell variant | Clear cell pattern with glycogen-rich cytoplasm | ||
| Plasmacytoid | Cells with abundant lipid content, mimic signet ring cell adenocarcinoma of stomach/ lobular breast cancer | Clinically aggressive, propensity for peritoneal spread | |
| Unusual stromal reactions | Presence of following; pseudosarcomatous stroma, stromal osseous or cartilaginous metaplasia, osteoclast-type giant cells, lymphoid infiltrate |
Staging
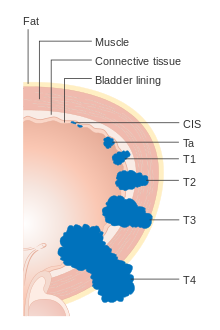




Bladder cancer is staged (classified by the extent of spread of the cancer) and graded (how abnormal and aggressive the cells appear under the microscope) to determine treatment and predict outcomes. Staging is usually performed with transurethral resection of bladder tumor (TURBT) and radiologic imaging (CT and MRI). Papillary tumors confined to the mucosa or which invade the lamina propria are classified as Ta or T1. Flat lesions that do not invade the basement membrane of the bladder mucosa are termed Tis (in situ). All three categories (Tis, Ta and T1) are grouped together as non-muscle invasive disease for therapeutic purposes and in most cases they are offered cystoscopic resection with TURBT without the need for radical resection of the entire urinary bladder. Tumors in the remaining categories (T2, T3 and T4) are termed muscle-invasive disease and are associated with less favorable prognosis.[56]
In the TNM staging system (8th Edn. 2017) for bladder cancer:[75][76]
T (Primary tumour)
- TX Primary tumour cannot be assessed
- T0 No evidence of primary tumour
- Ta Non-invasive papillary carcinoma
- Tis Carcinoma in situ ('flat tumour')
- T1 Tumour invades subepithelial connective tissue
- T2a Tumour invades superficial muscle (inner half of the detrusor muscle)[77]
- T2b Tumour invades deep muscle (outer half of the detrusor muscle)[77]
- T3 Tumour invades perivesical tissue:
- T3a Microscopically
- T3b Macroscopically (extravesical mass)
- T4a Tumour invades prostate, uterus or vagina
- T4b Tumour invades pelvic wall or abdominal wall
N (Lymph nodes)
- NX Regional lymph nodes cannot be assessed
- N0 No regional lymph node metastasis
- N1 Metastasis in a single lymph node in true pelvis (hypogastric, obturator, external iliac, or presacral nodes)
- N2 Metastasis in multiple lymph nodes in true pelvis (hypogastric, obturator, external iliac, or presacral nodes)
- N3 Metastasis in common iliac lymph nodes
M (Distant metastasis)
- MX Distant metastasis cannot be assessed
- M0 No distant metastasis
- M1 Distant metastasis.
- M1a: The cancer has spread only to lymph nodes outside of the pelvis.
- M1b: The cancer has spread other parts of the body.
The most common sites for bladder cancer metastases are the lymph nodes, bones, lung, liver, and peritoneum.[78] The most common sentinel lymph nodes draining bladder cancer are obturator and internal iliac lymph nodes. The location of lymphatic spread depends on the location of the tumors. Tumors on the superolateral bladder wall spread to external iliac lymph nodes. Tumors on the neck, anterior wall and fundus spread commonly to the internal iliac lymph nodes.[79] From the regional lymph nodes (i.e. obturator, internal and external lymph nodes) the cancer spreads to distant sites like the common iliac lymph nodes and paraaortic lymph nodes.[80] Skipped lymph node lesions are not seen in bladder cancer.[79]
Numerical
The stages above can be integrated into a numerical staging (with Roman numerals) as follows:[81]
| Stage | Tumor | Nodes | Metastasis | 5-year survival in the US[82] |
|---|---|---|---|---|
| Stage 0a | Ta | N0 | M0 | 98% |
| Stage 0is | Tis | N0 | M0 | 95% |
| Stage I | T1 | N0 | M0 | 63% |
| Stage II | T2a | N0 | M0 | |
| T2b | ||||
| Stage IIIA | T3a | N0 | M0 | 35% |
| T3b | ||||
| T4a | ||||
| T1-4a | N1 | |||
| Stage IIIB | T1-4a | N2 | M0 | |
| N3 | ||||
| Stage IVA | T4b | Any N | M0 | |
| Any T | M1a | |||
| Stage IVB | Any T | Any N | M1b | 5% |
Grading
According to WHO classification (1973) bladder cancers are histologically graded into:[83]
- G1 – Well differentiated,
- G2 – Moderately differentiated
- G3 – Poorly differentiated
WHO classification (2004/2016)[84][85]
- Papillary lesions
- Urothelial Papilloma
- Papillary urothelial neoplasm of low malignant potential (PUNLMP)
- Low Grade
- High Grade
- Flat lesions
- Urothelial proliferation of uncertain malignant potential
- Reactive atypia
- Atypia of unknown significance
- Urothelial dysplasia
- Urothelial CIS (always high grade)
- Primary
- Secondary
- Concurrent
Risk stratification
People with non-muscle invasive bladder cancer (NMIBC), are risk-stratified based on clinical and pathological factors so that they are treated appropriately depending on their probability of having progression and/or recurrence.[86] People with non-muscle invasive tumors are categorized into low-risk, intermediate-risk and high-risk or provided with a numerical risk score. Risk-stratification framework is provided by American Urology Association/Society of Urological Oncology (AUA/SUO stratification), European Association of Urology (EAU) guidelines, European Organization for Research and Treatment of Cancer (EORTC) risk tables and Club Urológico Español de Tratamiento Oncológico (CUETO) scoring model.[87][88][89]
| Low risk | Intermediate risk | High risk |
|---|---|---|
| Low grade solitary Ta tumor, smaller than 3 cm | Recurrence within 1 year, Low grade Ta tumor | High grade T1 |
| Papillary urothelial neoplasm of low malignant potential | Solitary low grade Ta tumor, bigger than 3 cm | Any recurrent tumor or any high grade Ta |
| Low grade Ta, multifocal tumors | High grade Ta, bigger than 3 cm (or multifocal) | |
| High grade Ta, smaller than 3 cm | Any carcinoma in situ | |
| Low grade T1 | Any BCG failure in high grade tumors | |
| Any variant histology | ||
| Any lymphovascular invasion | ||
| Any high grade prostatic urethral involvement |
The EORTC and CUETO model use a cumulative score obtained from individual prognostic factors, which are then converted into risk of progression and recurrence. The six prognostic factors included in the EORTC model are number of tumors, recurrence rate, T-stage, presence of carcinoma-in-situ and grade of the tumor. Scoring for recurrence in the CUETO model incorporates 6 variables; age, gender, grade, tumor status, number of tumors and presence of tis. For progression scoring the previous 6 variables plus T stage is used.[90][91]
| Model | Cumulative score for recurrence | Recurrence at 1-year (%) | Recurrence at 5-year (%) |
|---|---|---|---|
| EORTC | 0 | 15 | 31 |
| 1-4 | 24 | 46 | |
| 5-9 | 38 | 62 | |
| 10-17 | 61 | 78 | |
| CUETO | 0-4 | 8.2 | 21 |
| 5-6 | 12 | 36 | |
| 7-9 | 25 | 48 | |
| 10-16 | 42 | 68 |
| Model | Cumulative score for progression | Progression at 1-year (%) | Progression at 5-year (%) |
|---|---|---|---|
| EORTC | 0 | 0.2 | 0.8 |
| 2-6 | 1 | 6 | |
| 7-13 | 5 | 17 | |
| 12-23 | 17 | 45 | |
| CUETO | 0-4 | Zdroj:https://en.wikipedia.org?pojem=Bladder_carcinoma
