
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (October 2023) |
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities are on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the products to show the direction of the reaction.[1] The chemical formulas may be symbolic, structural (pictorial diagrams), or intermixed. The coefficients next to the symbols and formulas of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.[2]
Structure
A chemical equation (see an example below) consists of a list of reactants (the starting substances) on the left-hand side, an arrow symbol, and a list of products (substances formed in the chemical reaction) on the right-hand side. Each substance is specified by its chemical formula, optionally preceded by a number called stoichiometric coefficient.[a] The coefficient specifies how many entities (e.g. molecules) of that substance are involved in the reaction on a molecular basis. If not written explicitly, the coefficient is equal to 1. Multiple substances on any side of the equation are separated from each other by a plus sign.
As an example, the equation for the reaction of hydrochloric acid with sodium can be denoted:
- 2HCl + 2Na → 2NaCl + H2
Given the formulas are fairly simple, this equation could be read as "two H-C-L plus two N-A yields[b] two N-A-C-L and H two." Alternately, and in general for equations involving complex chemicals, the chemical formulas are read using IUPAC nomenclature, which could verbalise this equation as "two hydrochloric acid molecules and two sodium atoms react to form two formula units of sodium chloride and a hydrogen gas molecule."
Reaction types
Different variants of the arrow symbol are used to denote the type of a reaction:[1]
net forward reaction 
reaction in both directions[c] equilibrium[d] stoichiometric relation resonance (not a reaction)
State of matter
To indicate physical state of a chemical, a symbol in parentheses may be appended to its formula: (s) for a solid, (l) for a liquid, (g) for a gas, and (aq) for an aqueous solution. This is especially done when one wishes to emphasize the states or changes thereof. For example, the reaction of aqueous hydrochloric acid with solid (metallic) sodium to form aqueous sodium chloride and hydrogen gas would be written like this:
- 2HCl(aq) + 2Na(s) → 2NaCl(aq) + H2(g)
That reaction would have different thermodynamic and kinetic properties if gaseous hydrogen chloride were to replace the hydrochloric acid as a reactant:
- 2HCl(g) + 2Na(s) → 2NaCl(s) + H2(g)
Alternately, an arrow without parentheses is used in some cases to indicate formation of a gas ↑ or precipitate ↓. This is especially useful if only one such species is formed. Here is an example indicating that hydrogen gas is formed:
- 2HCl + 2Na → 2 NaCl + H2 ↑
Catalysis and other conditions
If the reaction requires energy, it is indicated above the arrow. A capital Greek letter delta (Δ) or a triangle (△)[e] is put on the reaction arrow to show that energy in the form of heat is added to the reaction. The expression hν[f] is used as a symbol for the addition of energy in the form of light. Other symbols are used for other specific types of energy or radiation.
Similarly, if a reaction requires a certain medium with certain specific characteristics, then the name of the acid or base that is used as a medium may be placed on top of the arrow. If no specific acid or base is required, another way of denoting the use of an acidic or basic medium is to write H+ or OH− (or even "acid" or "base") on top of the arrow. Specific conditions of the temperature and pressure, as well as the presence of catalysts, may be indicated in the same way.
Notation variants

The standard notation for chemical equations only permits all reactants on one side, all products on the other, and all stoichiometric coefficients positive. For example, the usual form of the equation for dehydration of methanol to dimethylether is:
- 2 CH3OH → CH3OCH3 + H2O
Sometimes an extension is used, where some substances with their stoichiometric coefficients are moved above or below the arrow, preceded by a plus sign or nothing for a reactant, and by a minus sign for a product. Then the same equation can look like this:
Such notation serves to hide less important substances from the sides of the equation, to make the type of reaction at hand more obvious, and to facilitate chaining of chemical equations. This is very useful in illustrating multi-step reaction mechanisms. Note that the substances above or below the arrows are not catalysts in this case, because they are consumed or produced in the reaction like ordinary reactants or products.
Another extension used in reaction mechanisms moves some substances to branches of the arrow. Both extensions are used in the example illustration of a mechanism.
Use of negative stoichiometric coefficients at either side of the equation (like in the example below) is not widely adopted and is often discouraged.[5][better source needed]
Balancing chemical equations
Because no nuclear reactions take place in a chemical reaction, the chemical elements pass through the reaction unchanged. Thus, each side of the chemical equation must represent the same number of atoms of any particular element (or nuclide, if different isotopes are taken into account). The same holds for the total electric charge, as stated by the charge conservation law. An equation adhering to these requirements is said to be balanced.
A chemical equation is balanced by assigning suitable values to the stoichiometric coefficients. Simple equations can be balanced by inspection, that is, by trial and error. Another technique involves solving a system of linear equations.
Balanced equations are usually written with smallest natural-number coefficients. Yet sometimes it may be advantageous to accept a fractional coefficient, if it simplifies the other coefficients. The introductory example can thus be rewritten as
In some circumstances the fractional coefficients are even inevitable. For example, the reaction corresponding to the standard enthalpy of formation must be written such that one molecule of a single product is formed. This will often require that some reactant coefficients be fractional, as is the case with the formation of lithium fluoride:
Inspection method
4 + 2 O
2 → CO
2 + 2 H
2O, a coefficient of 2 must be placed before the oxygen gas on the reactants side and before the water on the products side in order for, as per the law of conservation of mass, the quantity of each element does not change during the reaction
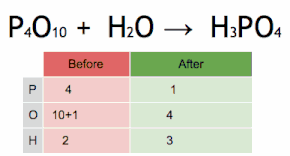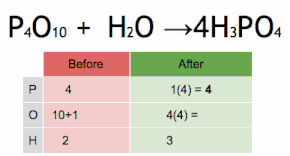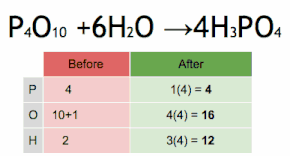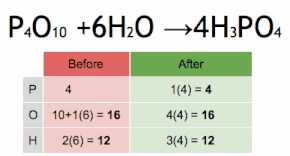
This chemical equation is being balanced by first multiplying H3PO4 by four to match the number of P atoms, and then multiplying H2O by six to match the numbers of H and O atoms.
The method of inspection can be outlined as setting the most complex substance's stoichiometric coefficient to 1 and assigning values to other coefficients step by step such that both sides of the equation end up with the same number of atoms for each element. If any fractional coefficients arise during this process, the presence of fractions may be eliminated (at any time) by multiplying all coefficients by their lowest common denominator.
- Example
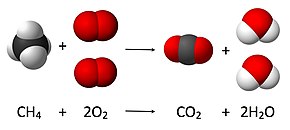
Balancing of the chemical equation for the complete combustion of methane
is achieved as follows:
- A coefficient of 1 is placed in front of the most complex formula (CH4):
- The left-hand side has 1 carbon atom, so 1 molecule of CO2 will balance it. The left-hand side also has 4 hydrogen atoms, which will be balanced by 2 molecules of H2O:
- Balancing the 4 oxygen atoms of the right-hand side by 2 molecules of O2 yields the equation
- The coefficients equal to 1 are omitted, as they do not need to be specified explicitly:
- It is wise to check that the final equation is balanced, i.e. that for each element there is the same number of atoms on the left- and right-hand side: 1 carbon, 4 hydrogen, and 4 oxygen.
System of linear equations
For each chemical element (or nuclide or unchanged moiety or charge) i, its conservation requirement can be expressed by the mathematical equation
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk













