
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.[1]
Bonding
Halides are X-type ligands in coordination chemistry. They are both σ- and π-donors. Chloride is commonly found as both a terminal ligand and a bridging ligand. The halide ligands are weak field ligands. Due to a smaller crystal field splitting energy, the homoleptic halide complexes of the first transition series are all high spin. Only 3− is exchange inert.
Homoleptic metal halide complexes are known with several stoichiometries, but the main ones are the hexahalometallates and the tetrahalometallates. The hexahalides adopt octahedral coordination geometry, whereas the tetrahalides are usually tetrahedral. Square planar tetrahalides are known for Pd(II), Pt(II), and Au(III). Examples with 2- and 3-coordination are common for Au(I), Cu(I), and Ag(I).
Due to the presence of filled pπ orbitals, halide ligands on transition metals are able to reinforce π-backbonding onto a π-acid. They are also known to labilize cis-ligands.[2] [3]
Homoleptic complexes
Homoleptic complexes (complexes with only chloride ligands) are often common reagents. Almost all examples are anions.
1st row
| Complex | colour | electron config. | structure | geometry | comments |
|---|---|---|---|---|---|
| TiCl4 | colourless | (t2g)0 | tetrahedral | ||
| − | white/colourless | d0d0 | face-sharing bioctahedron | Ti-Cl(terminal) = 2.23 Å, 2.45 (terminal) (N(PCl3)2)+ salt)[4] | |
| 3- | orange | (t2g)1(t2g)1 | face-sharing bioctahedron | Ti-Ti =3.22 Å Ti-C1(terminal) = 2.32-2.35 Å, Ti-Cl(bridge) = 2.42-2.55 Å ((NEt4+)3)3 salt)[5] | |
| 2− | colourless | d0d0 | 
|
bioctahedral | |
| 3- | green | (t2g)1(t2g)1(t2g)1 | face-sharing trioctahedron | Ti-Ti = 3.19, 3.10 Å (terminal) Ti-C1(terminal) = 2.36 Å (terminal), Ti-Cl(bridge) = 2.50 Å ((PPh4+)3)3 salt)[6] | |
| 2− | yellow | d0 | 
|
octahedral | PPh4+ salt Ti-Cl = 2.33 Å[7] |
| VCl4 | red | (t2g)1 | tetrahedral | V1−Cl = 2.29 Å | |
| V2Cl10 | violet | (t2g)0 | 
|
edge-shared bioctahedron | V1−Cl(bridging) = 2.48 Å V1−Cl(terminal) = 2.16-2.21 Å[8] |
| 2- | red | (t2g)1 | 
|
octahedral | V1−Cl = 2.29 Å[9] |
| 3− | pink[3] | (t2g)3 | 
|
octahedral[10][3] | |
| 3− | red | (d3)2 | face-sharing bioctahedron | Cr-Cl(terminal) = 2.31 Å, 2.42 (terminal) (Et2NH2+ salt)[11] | |
| 2−[12] | pale pink to while | (eg)2(t2g)3 | tetrahedral | Mn-Cl bond length = 2.3731-2.3830 Å[13] | |
| 2− | dark red | (t2g)3(eg)1 | 
|
octahedral | Mn-Cl distance = 2.28 Å K+ salt[14]) salt is isostructural with K2PtCl6 |
| 3− | brown[3] | (t2g)3(eg)1 | 
|
octahedral[10][3] | |
| 2− | yellow-green | (eg)2(t2g)3 | 
|
bitetrahedral | Mn-Cl(terminal) bond length = 2.24 Å Mn-Cl(terminal) bond length = 2.39 Å[15] (PPN+)2 salt |
| 6− | pink | (t2g)3(eg)2 | cofacial trioctahedron | Mn-Cl distance = --- Å +6 salt[16] | |
| 2−[12] | cream | (eg)3(t2g)3 | tetrahedral((Et4N+)2 salt)[12] | ||
| − | (eg)2(t2g)3 | tetrahedral | Fe-Cl bond length = 2.19 Å[17] | ||
| 3− | orange | (t2g)3(eg)2 | 
|
octahedral[3] | |
| Fe2Cl62− | pale yellow | (eg)2(t2g)3 | 
|
bitetrahedral | Fe-Cl(terminal) bond length = 2.24 Å Fe-Cl(terminal) bond length = 2.39 Å[15] (PPN+)2 salt |
| CoCl42−[12] | blue[12] | (eg)4(t2g)3 | tetrahedral | ||
| Co2Cl62− | blue[15] | (eg)4(t2g)3 | 
|
bitetrahedral | Mn-Cl(terminal) bond length = 2.24 Å Co-Cl(terminal) bond length = 2.35 Å[15] (PPN+)2 salt |
| NiCl42−[12] | blue[12] | (eg)4(t2g)4 | tetrahedral | Ni-Cl bond length = 2.28 Å (Et4N+)2 salt[18] | |
| Ni3Cl126− | orange[19] | (t2g)6(eg)2 | confacial trioctahedral | ((Me2NH2+)2)8 salt double salt with two Cl− Ni-Cl bond length = 2.36-2.38 Å[19] | |
| CuCl42−[12] | orange[20] yellow (flattened tetrahedral)[21] green (square planar)[22] |
(t2g)6(eg)3 | flattened tetrahedral or square planar[23][24] |
Cu-Cl bond length = 2.24 Å | |
| Cu2Cl62− | red | (t2g)6(eg)32 | edge-shared bis(square planar)[25] | Cu-Cl(terminal) = 2.24 Å Cu-Cl(bridging) = 2.31 Å | |
| ZnCl42− | white/colorless | d10 | tetrahedral |
2nd rowedit
Some homoleptic complexes of the second row transition metals feature metal-metal bonds.
| Complex | colour | electron config. | structure | geometry | comments |
|---|---|---|---|---|---|
| ZrCl62− | yellow | d0 | 
|
octahedral | Zr-Cl distance = 2.460 Å (Me4N+)2 salt[27] |
| Zr2Cl102− | colorless | (d0)2 | 
|
edge-shared bioctahedral | Zr-Cl = 2.36 Å (terminal), 2.43 Å (bridging) N(PCl3)2)+ salt[4] |
| Nb2Cl10 | yellow | (d0)2 | 
|
edge-shared bioctahedral Nb2Cl10 | 3.99 Å[28] |
| NbCl6− | yellow | d0 | 
|
octahedral | Nb-Cl = 2.34 Å N(PCl3)2)+ salt[4] |
| Nb6Cl182− | black | (d2)4(d3)2 (14 cluster electrons) | 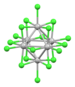
|
cluster Nb---Nb bonding | Nb-Cl = 2.92 Å (K+)2 salt[29] |
| MoCl6 | black | d0 | 
|
octahedron | Mo−Cl = 2.28 -2.31 Å[8] |
| MoCl62− | yellow | (t2g)2 | 
|
octahedron | Mo−Cl = 2.37, 2.38, 2.27 Å[30] |
| MoCl63− | pink | (t2g)3 | 
|
octahedral | |
| Mo2Cl84− | purple[31] | 2(d4) | 
|
Mo-Mo quadruple bond | |
| Mo2Cl93− | 2(d3) | face-shared bioctahedral | Mo-Mo (triple) bond length = 2.65 Å Mo-Cl (terminal) bond length = 2.38 Å Mo-Cl (bridging) bond length = 2.49 Å[32][33] | ||
| Mo2Cl10 | green | (d1)2 | 
|
edge-sharing bioctahedra[34] | |
| Mo2Cl102− | (d2)2 | 
|
edge-sharing bioctahedra[35] | ||
| Mo5Cl132− | brown[31] | d2d2d2d2d3 | 
|
incomplete octahedron[36] | |
| Mo6Cl142− | yellow | d4 | 
|
octahedral cluster | (4-HOPyH+)2 salt[37] |
| TcCl62− | yellow | (t2g)3 | 
|
octahedron | Tc-Cl = 2.35 Å for As(C6H5)4+ salt[38] |
| Tc2Cl82− | green | (t2g)4 | 
|
Tc-Tc quadruple bond | Tc-Tc = 2.16, Tc-Cl = 2.34 Å for NBu4+ salt[39] |
| RuCl62− | brown | (t2g)4 | 
|
octahedral | (EtPPh3+)2 salt[40] |
| Ru2Cl93− | red | (t2g)52 | cofacial bioctahedral | Ru-Ru bond length = 2.71 Å; Ru-Cl(terminal) = 2.35 Å, Ru-Cl(bridging) = 2.36 Å ((Et4N)+)3 salt[41] | |
| Ru3Cl124− | green | (d5)2(d6) | cofacial trioctahedral | Ru-Ru bond lengths = 2.86 Å Ru-Cl bond lengths = 2.37-2.39 Å (Et4N+)2(H7O3+)2 salt[42] | |
| RhCl63− | red | (t2g)6 | 
|
octahedral | H2N+(CH2CH2NH3+)2 salt)[43] |
| Rh2Cl93− | red-brown | (t2g)6 | octahedral | Rh-Cl(terminal) = 2.30 Å, Rh-Cl(terminal) = 2.40 Å ((Me3CH2Ph)+)3 salt)[32] | |
| PdCl42− | brown | d8 | square planar | ||
| Pd2Cl62−[44] | red ((Et4N+)2 salt) | d8 | square planar | ||
| Pd3Cl82−[45] | orange brown ((Bu4N+)2 salt) | d8 | square planar | ||
| PdCl62− | brown | d6 | 
|
octahedral | Pd(IV) |
| Pd6Cl12 | yellow-brown | d8 | 
|
square planar[46] | |
| AgCl2− | white/colorless | d10 | linear | salt of K(2.2.2-crypt)+[47] | |
| CdCl42− | white/colorless | d10 | tetrahedral | Et4N+ salt, Cd-Cl distance is 2.43 Å[26] | |
| Cd2Cl62− | white/colorless | d10 | 
|
edge-shared bitetrahedron | (C6N3(4-C5H4N)33+ salt[48] |
| Cd3Cl126− | white/colorless | d10 | octahedral (central Cd) pentacoordinate (terminal Cd's) cofactial trioctahedral |
(C6N3(4-C5H4N)33+ salt[48] (3,8-Diammonium-6-phenylphenanthridine3+)2[49] | |
| Cd6Cl197− | white/colorless | d10 | 
|
octahedron of octahedra | 4,4'-(C6H3(2-Et)NH3+)2 salt[50] |
