
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

| |

| |
| Names | |
|---|---|
| IUPAC name | |
| Systematic IUPAC name
(1S,3aS,3bS,9aR,9bS,11aS)-1-Acetyl-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopentaphenanthren-7-one | |
| Other names
P4;[1] Pregnenedione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.318 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H30O2 | |
| Molar mass | 314.469 g/mol |
| Melting point | 126 |
| log P | 4.04[4] |
| Pharmacology | |
| G03DA04 (WHO) | |
| By mouth, topical/transdermal, vaginal, intramuscular injection, subcutaneous injection, subcutaneous implant | |
| Pharmacokinetics: | |
| OMP: <10%[5][6] | |
| • Albumin: 80% • CBG: 18% • SHBG: <1% • Free: 1–2%[7][8] | |
| Hepatic (CYP2C19, CYP3A4, CYP2C9, 5α-reductase, 3α-HSD, 17α-hydroxylase, 21-hydroxylase, 20α-HSD)[9][10] | |
| OMP: 16–18 hours[5][6][11] IM: 22–26 hours[6][12] SC: 13–18 hours[12] | |
| Renal | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species.[1][13] It belongs to a group of steroid hormones called the progestogens[13] and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.[14]
In addition to its role as a natural hormone, progesterone is also used as a medication, such as in combination with estrogen for contraception, to reduce the risk of uterine or cervical cancer, in hormone replacement therapy, and in feminizing hormone therapy.[15] It was first prescribed in 1934.[16]
Biological activity
Progesterone is the most important progestogen in the body. As a potent agonist of the nuclear progesterone receptor (nPR) (with an affinity of KD = 1 nM) the resulting effects on ribosomal transcription plays a major role in regulation of female reproduction.[13][17] In addition, progesterone is an agonist of the more recently discovered membrane progesterone receptors (mPRs),[18] of which the expression has regulation effects in reproduction function (oocyte maturation, labor, and sperm motility) and cancer although additional research is required to further define the roles.[19] It also functions as a ligand of the PGRMC1 (progesterone receptor membrane component 1) which impacts tumor progression, metabolic regulation, and viability control of nerve cells.[20][21][22] Moreover, progesterone is also known to be an antagonist of the sigma σ1 receptor,[23][24] a negative allosteric modulator of nicotinic acetylcholine receptors,[14] and a potent antagonist of the mineralocorticoid receptor (MR).[25] Progesterone prevents MR activation by binding to this receptor with an affinity exceeding even those of aldosterone and glucocorticoids such as cortisol and corticosterone,[25] and produces antimineralocorticoid effects, such as natriuresis, at physiological concentrations.[26] In addition, progesterone binds to and behaves as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol).[27][28]
Progesterone, through its neurosteroid active metabolites such as 5α-dihydroprogesterone and allopregnanolone, acts indirectly as a positive allosteric modulator of the GABAA receptor.[29]
Progesterone and some of its metabolites, such as 5β-dihydroprogesterone, are agonists of the pregnane X receptor (PXR),[30] albeit weakly so (EC50 >10 μM).[31] In accordance, progesterone induces several hepatic cytochrome P450 enzymes,[32] such as CYP3A4,[33][34] especially during pregnancy when concentrations are much higher than usual.[35] Perimenopausal women have been found to have greater CYP3A4 activity relative to men and postmenopausal women, and it has been inferred that this may be due to the higher progesterone levels present in perimenopausal women.[33]
Progesterone modulates the activity of CatSper (cation channels of sperm) voltage-gated Ca2+ channels. Since eggs release progesterone, sperm may use progesterone as a homing signal to swim toward eggs (chemotaxis). As a result, it has been suggested that substances that block the progesterone binding site on CatSper channels could potentially be used in male contraception.[36][37]
Biological function

Hormonal interactions
Progesterone has a number of physiological effects that are amplified in the presence of estrogens. Estrogens through estrogen receptors (ERs) induce or upregulate the expression of the PR.[39] One example of this is in breast tissue, where estrogens allow progesterone to mediate lobuloalveolar development.[40][41][42]
Elevated levels of progesterone potently reduce the sodium-retaining activity of aldosterone, resulting in natriuresis and a reduction in extracellular fluid volume. Progesterone withdrawal, on the other hand, is associated with a temporary increase in sodium retention (reduced natriuresis, with an increase in extracellular fluid volume) due to the compensatory increase in aldosterone production, which combats the blockade of the mineralocorticoid receptor by the previously elevated level of progesterone.[43]
Early sexual differentiation
Progesterone plays a role in early human sexual differentiation.[44] Placental progesterone is the feedstock for the 5α-dihydrotestosterone (DHT) produced via the backdoor pathway found operating in multiple non-gonadal tissues of the fetus,[45] whereas deficiencies in this pathway lead to undervirilization of the male fetus, resulting in incomplete development of the male genitalia.[46][47] DHT is a potent androgen that is responsible for the development of male genitalia, including the penis and scrotum.
During early fetal development, the undifferentiated gonads can develop into either testes or ovaries. The presence of the Y chromosome leads to the development of testes. The testes then produce testosterone, which is converted to DHT via the enzyme 5α-reductase. DHT is a potent androgen that is responsible for the masculinization of the external genitalia and the development of the prostate gland. Progesterone, produced by the placenta during pregnancy, plays a role in fetal sexual differentiation by serving as a precursor molecule for the synthesis of DHT via the backdoor pathway. In the absence of adequate levels of steroidogenic enzymes during fetal development, the backdoor pathway for DHT synthesis can become deficient, leading to undermasculinization of the male fetus. This can result in the development of ambiguous genitalia or even female genitalia in some cases. Therefore, both DHT and progesterone play crucial roles in early fetal sexual differentiation, with progesterone acting as a precursor molecule for DHT synthesis and DHT promoting the development of male genitalia.[44]
Reproductive system
Progesterone has key effects via non-genomic signalling on human sperm as they migrate through the female tract before fertilization occurs, though the receptor(s) as yet remain unidentified.[48] Detailed characterisation of the events occurring in sperm in response to progesterone has elucidated certain events including intracellular calcium transients and maintained changes,[49] slow calcium oscillations,[50] now thought to possibly regulate motility.[51] It is produced by the ovaries.[52] Progesterone has also been shown to demonstrate effects on octopus spermatozoa.[53]
Progesterone is sometimes called the "hormone of pregnancy",[54] and it has many roles relating to the development of the fetus:
- Progesterone converts the endometrium to its secretory stage to prepare the uterus for implantation. At the same time progesterone affects the vaginal epithelium and cervical mucus, making it thick and impenetrable to sperm. Progesterone is anti-mitogenic in endometrial epithelial cells, and as such, mitigates the tropic effects of estrogen.[55] If pregnancy does not occur, progesterone levels will decrease, leading to menstruation. Normal menstrual bleeding is progesterone-withdrawal bleeding. If ovulation does not occur and the corpus luteum does not develop, levels of progesterone may be low, leading to anovulatory dysfunctional uterine bleeding.
- During implantation and gestation, progesterone appears to decrease the maternal immune response to allow for the acceptance of the pregnancy.[56]
- Progesterone decreases contractility of the uterine smooth muscle.[54] This effect contributes to prevention of preterm labor.[56] Studies have shown that in women who are pregnant with a single fetus, asymptomatic in the prenatal stage, and at a high risk of giving pre-term birth spontaneously, vaginal progesterone medication has been found to be effective in preventing spontaneous pre-term birth. Women who are at a high risk of giving pre-term birth spontaneously are those who have a short cervix of less than 25 mm or have previously given pre-term birth spontaneously. Although pre-term births are generally considered to be less than 37 weeks, these studies found that vaginal progesterone is associated with fewer pre-term births of less than 34 weeks.[57]
- A drop in progesterone levels is possibly one step that facilitates the onset of labor.
- In addition, progesterone inhibits lactation during pregnancy. The fall in progesterone levels following delivery is one of the triggers for milk production.
The fetus metabolizes placental progesterone in the production of adrenal steroids.[45]
Breasts
Lobuloalveolar development
Progesterone plays an important role in breast development in women. In conjunction with prolactin, it mediates lobuloalveolar maturation of the mammary glands during pregnancy to allow for milk production and thus lactation and breastfeeding of offspring following parturition (childbirth).[58] Estrogen induces expression of the PR in breast tissue and hence progesterone is dependent on estrogen to mediate lobuloalveolar development.[40][41][42] It has been found that RANKL is a critical downstream mediator of progesterone-induced lobuloalveolar maturation.[59] RANKL knockout mice show an almost identical mammary phenotype to PR knockout mice, including normal mammary ductal development but complete failure of the development of lobuloalveolar structures.[59]
Ductal development
Though to a far lesser extent than estrogen, which is the major mediator of mammary ductal development (via the ERα),[60][61] progesterone may be involved in ductal development of the mammary glands to some extent as well.[62] PR knockout mice or mice treated with the PR antagonist mifepristone show delayed although otherwise normal mammary ductal development at puberty.[62] In addition, mice modified to have overexpression of PRA display ductal hyperplasia,[59] and progesterone induces ductal growth in the mouse mammary gland.[62] Progesterone mediates ductal development mainly via induction of the expression of amphiregulin, the same growth factor that estrogen primarily induces the expression of to mediate ductal development.[62] These animal findings suggest that, while not essential for full mammary ductal development, progesterone seems to play a potentiating or accelerating role in estrogen-mediated mammary ductal development.[62]
Breast cancer risk
Progesterone also appears to be involved in the pathophysiology of breast cancer, though its role, and whether it is a promoter or inhibitor of breast cancer risk, has not been fully elucidated.[63][64] Most progestins, or synthetic progestogens, like medroxyprogesterone acetate, have been found to increase the risk of breast cancer in postmenopausal women in combination with estrogen as a component of menopausal hormone therapy.[65][64] The combination of natural oral progesterone or the atypical progestin dydrogesterone with estrogen has been associated with less risk of breast cancer than progestins plus estrogen.[66][67][68] However, this may simply be an artifact of the low progesterone levels produced with oral progesterone.[63][69] More research is needed on the role of progesterone in breast cancer.[64]
Skin health
The estrogen receptor, as well as the progesterone receptor, have been detected in the skin, including in keratinocytes and fibroblasts.[70][71] At menopause and thereafter, decreased levels of female sex hormones result in atrophy, thinning, and increased wrinkling of the skin and a reduction in skin elasticity, firmness, and strength.[70][71] These skin changes constitute an acceleration in skin aging and are the result of decreased collagen content, irregularities in the morphology of epidermal skin cells, decreased ground substance between skin fibers, and reduced capillaries and blood flow.[70][71] The skin also becomes more dry during menopause, which is due to reduced skin hydration and surface lipids (sebum production).[70] Along with chronological aging and photoaging, estrogen deficiency in menopause is one of the three main factors that predominantly influences skin aging.[70]
Hormone replacement therapy, consisting of systemic treatment with estrogen alone or in combination with a progestogen, has well-documented and considerable beneficial effects on the skin of postmenopausal women.[70][71] These benefits include increased skin collagen content, skin thickness and elasticity, and skin hydration and surface lipids.[70][71] Topical estrogen has been found to have similar beneficial effects on the skin.[70] In addition, a study has found that topical 2% progesterone cream significantly increases skin elasticity and firmness and observably decreases wrinkles in peri- and postmenopausal women.[71] Skin hydration and surface lipids, on the other hand, did not significantly change with topical progesterone.[71]
These findings suggest that progesterone, like estrogen, also has beneficial effects on the skin, and may be independently protective against skin aging.[71]
Sexuality
Libido
Progesterone and its neurosteroid active metabolite allopregnanolone appear to be importantly involved in libido in females.[72]
Homosexuality
Dr. Diana Fleischman, of the University of Portsmouth, and colleagues looked for a relationship between progesterone and sexual attitudes in 92 women. Their research, published in the Archives of Sexual Behavior found that women who had higher levels of progesterone scored higher on a questionnaire measuring homoerotic motivation. They also found that men who had high levels of progesterone were more likely to have higher homoerotic motivation scores after affiliative priming compared to men with low levels of progesterone.[73][74][75][76]
Nervous system
Progesterone, like pregnenolone and dehydroepiandrosterone (DHEA), belongs to an important group of endogenous steroids called neurosteroids. It can be metabolized within all parts of the central nervous system.[77]
Neurosteroids are neuromodulators, and are neuroprotective, neurogenic, and regulate neurotransmission and myelination.[78] The effects of progesterone as a neurosteroid are mediated predominantly through its interactions with non-nuclear PRs, namely the mPRs and PGRMC1, as well as certain other receptors, such as the σ1 and nACh receptors.[79]
Brain damage
Previous studies have shown that progesterone supports the normal development of neurons in the brain, and that the hormone has a protective effect on damaged brain tissue. It has been observed in animal models that females have reduced susceptibility to traumatic brain injury and this protective effect has been hypothesized to be caused by increased circulating levels of estrogen and progesterone in females.[80]
Proposed mechanism
The mechanism of progesterone protective effects may be the reduction of inflammation that follows brain trauma and hemorrhage.[81][82]
Damage incurred by traumatic brain injury is believed to be caused in part by mass depolarization leading to excitotoxicity. One way in which progesterone helps to alleviate some of this excitotoxicity is by blocking the voltage-dependent calcium channels that trigger neurotransmitter release.[83] It does so by manipulating the signaling pathways of transcription factors involved in this release. Another method for reducing the excitotoxicity is by up-regulating the GABAA, a widespread inhibitory neurotransmitter receptor.[84]
Progesterone has also been shown to prevent apoptosis in neurons, a common consequence of brain injury.[85] It does so by inhibiting enzymes involved in the apoptosis pathway specifically concerning the mitochondria, such as activated caspase 3 and cytochrome c.
Not only does progesterone help prevent further damage, it has also been shown to aid in neuroregeneration.[86] One of the serious effects of traumatic brain injury includes edema. Animal studies show that progesterone treatment leads to a decrease in edema levels by increasing the concentration of macrophages and microglia sent to the injured tissue.[83][87] This was observed in the form of reduced leakage from the blood brain barrier in secondary recovery in progesterone treated rats. In addition, progesterone was observed to have antioxidant properties, reducing the concentration of oxygen free radicals faster than without.[84] There is also evidence that the addition of progesterone can also help remyelinate damaged axons due to trauma, restoring some lost neural signal conduction.[84] Another way progesterone aids in regeneration includes increasing the circulation of endothelial progenitor cells in the brain.[88] This helps new vasculature to grow around scar tissue which helps repair the area of insult.
Addictionedit
Progesterone enhances the function of serotonin receptors in the brain, so an excess or deficit of progesterone has the potential to result in significant neurochemical issues. This provides an explanation for why some people resort to substances that enhance serotonin activity such as nicotine, alcohol, and cannabis when their progesterone levels fall below optimal levels.[89]
- Sex differences in hormone levels may induce women to respond differently than men to nicotine. When women undergo cyclic changes or different hormonal transition phases (menopause, pregnancy, adolescence), there are changes in their progesterone levels.[90] Therefore, females have an increased biological vulnerability to nicotine's reinforcing effects compared to males and progesterone may be used to counter this enhanced vulnerability. This information supports the idea that progesterone can affect behavior.[89]
- Similar to nicotine, cocaine also increases the release of dopamine in the brain. The neurotransmitter is involved in the reward center and is one of the main neurotransmitters involved with substance abuse and reliance. In a study of cocaine users, it was reported that progesterone reduced craving and the feeling of being stimulated by cocaine. Thus, progesterone was suggested as an agent that decreases cocaine craving by reducing the dopaminergic properties of the drug.[91]
Societaledit
In a 2012 University of Amsterdam study of 120 women, women's luteal phase (higher levels of progesterone, and increasing levels of estrogen) was correlated with lower level of competitive behavior in gambling and math contest scenarios, while their premenstrual phase (sharply-decreasing levels of progesterone, and decreasing levels of estrogen) was correlated with a higher level of competitive behavior.[92]
Other effectsedit
- Progesterone also has a role in skin elasticity and bone strength, in respiration, in nerve tissue and in female sexuality, and the presence of progesterone receptors in certain muscle and fat tissue may hint at a role in sexually dimorphic proportions of those.[93][infringing link?]
- During pregnancy, progesterone is said to decrease uterine irritability.[94]
- During pregnancy, progesterone helps to suppress immune responses of the mother to fetal antigens, which prevents rejection of the fetus.[94]
- Progesterone raises epidermal growth factor-1 (EGF-1) levels, a factor often used to induce proliferation, and used to sustain cultures, of stem cells.[95]
- Progesterone increases core temperature (thermogenic function) during ovulation.[96][97]
- Progesterone reduces spasm and relaxes smooth muscle. Bronchi are widened and mucus regulated. (PRs are widely present in submucosal tissue.)
- Progesterone acts as an antiinflammatory agent and regulates the immune response.
- Progesterone reduces gall-bladder activity.[98]
- Progesterone normalizes blood clotting and vascular tone, zinc and copper levels, cell oxygen levels, and use of fat stores for energy.
- Progesterone may affect gum health, increasing risk of gingivitis (gum inflammation).[99]
- Progesterone appears to prevent endometrial cancer (involving the uterine lining) by regulating the effects of estrogen.
- Progesterone plays an important role in the signaling of insulin release and pancreatic function, and may affect the susceptibility to diabetes or gestational diabetes.[100][101]
- Progesterone levels in the blood were found to be lower in women who had higher weight and higher BMI among those who became pregnant through in vitro fertilization.[102]
- Current data shows that micronized progesterone, which is chemically identical to the progesterone produced in women's bodies, in combination with estrogen in menopausal hormone therapy does not seem to have significant effects on venous thromboembolism (blood clots in veins) and ischemic stroke (lack of blood flow to the brain due to blockage of a blood vessel that supplies the brain). However, more studies need to be conducted to see whether or not micronized progesterone alone or in combined menopausal hormone therapy changes the risk of myocardial infarctions (heart attacks).[103]
- There have not been any studies done yet on the effects of micronized progesterone on hair loss due to menopause.[104]
- Despite suggestions for using hormone therapy to prevent loss of muscle mass in post-menopausal women (50 and older), menopausal hormone therapy involving either estrogen alone or estrogen and progesterone has not been found to preserve muscle mass.[105] Menopausal hormone therapy also does not result in body weight reduction, BMI reduction, or change in glucose metabolism.[106]
Biochemistryedit
Biosynthesisedit
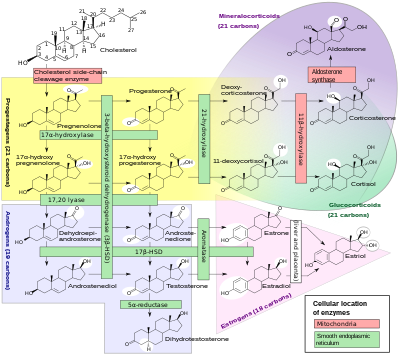
In mammals, progesterone, like all other steroid hormones, is synthesized from pregnenolone, which itself is derived from cholesterol.
Cholesterol undergoes double oxidation to produce 22R-hydroxycholesterol and then 20α,22R-dihydroxycholesterol. This vicinal diol is then further oxidized with loss of the side chain starting at position C22 to produce pregnenolone. This reaction is catalyzed by cytochrome P450scc.
The conversion of pregnenolone to progesterone takes place in two steps. First, the 3β-hydroxyl group is oxidized to a keto group and second, the double bond is moved to C4, from C5 through a keto/enol tautomerization reaction.[108] This reaction is catalyzed by 3β-hydroxysteroid dehydrogenase/δ5-4-isomerase.
Progesterone in turn is the precursor of the mineralocorticoid aldosterone, and after conversion to 17α-hydroxyprogesterone, of cortisol and androstenedione. Androstenedione can be converted to testosterone, estrone, and estradiol, highlighting the critical role of progesterone in testosterone synthesis.
Pregnenolone and progesterone can also be synthesized by yeast.[109]
Approximately 25 mg of progesterone is secreted from the ovaries per day in women, while the adrenal glands produce about 2 mg of progesterone per day.[110]
| Sex | Sex hormone | Reproductive phase |
Blood production rate |
Gonadal secretion rate |
Metabolic clearance rate |
Reference range (serum levels) | |
|---|---|---|---|---|---|---|---|
| SI units | Non-SI units | ||||||
| Men | Androstenedione | –
|
2.8 mg/day | 1.6 mg/day | 2200 L/day | 2.8–7.3 nmol/L | 80–210 ng/dL |
| Testosterone | –
|
6.5 mg/day | 6.2 mg/day | 950 L/day | 6.9–34.7 nmol/L | 200–1000 ng/dL | |
| Estrone | –
|
150 μg/day | 110 μg/day | 2050 L/day | 37–250 pmol/L | 10–70 pg/mL | |
| Estradiol | –
|
60 μg/day | 50 μg/day | 1600 L/day | <37–210 pmol/L | 10–57 pg/mL | |
| Estrone sulfate | –
|
80 μg/day | Insignificant | 167 L/day | 600–2500 pmol/L | 200–900 pg/mL | |
| Women | Androstenedione | –
|
3.2 mg/day | 2.8 mg/day | 2000 L/day | 3.1–12.2 nmol/L | 89–350 ng/dL |
| Testosterone | –
|
190 μg/day | 60 μg/day | 500 L/day | 0.7–2.8 nmol/L | 20–81 ng/dL | |
| Estrone | Follicular phase | 110 μg/day | 80 μg/day | Zdroj:https://en.wikipedia.org?pojem=Progesterone||||
