
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Furan-2-yl)methanethiol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 383594 | |
| ChemSpider | |
| ECHA InfoCard | 100.002.390 |
| EC Number |
|
| MeSH | furfuryl+mercaptan |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3336 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H6OS | |
| Molar mass | 114.16 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Roasted coffee, Caramel, Sulfurous, Waxy |
| Density | 1.132 g cm−3 |
| Boiling point | 155 °C; 311 °F; 428 K |
| Vapor pressure | 531 Pa |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H226 | |
| Flash point | 45 °C (113 °F; 318 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
100-200 mg kg−1 (mouse) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
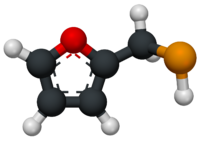
Furan-2-ylmethanethiol (2-Furanmethanethiol) is an organic compound containing a furan substituted with a sulfanylmethyl group. It is a clear colourless liquid when pure, but it becomes yellow coloured upon prolonged standing. It possesses a strong odour of roasted coffee and a bitter taste. It is a key component of the aroma of roasted coffee. It has been identified as a trigger molecule for parosmia following COVID-19 infection.[1][2]
Synthesis
Furan-2-ylmethanethiol is easily prepared by reacting furfuryl alcohol with thiourea in hydrochloric acid via an intermediate isothiouronium salt which is hydrolized to the thiol by heating with sodium hydroxide.[3]
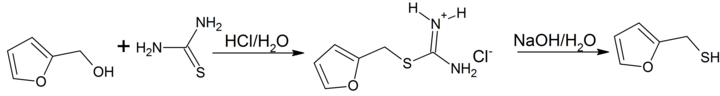
References
- ^ Parker JK, Kelly CE, Gane SB (5 February 2021). "Molecular Mechanism of Parosmia". p. 21251085. medRxiv 10.1101/2021.02.05.21251085.
- ^ Devlin H (25 May 2022). "Scientists identify 'trigger molecule' for Covid-related changes to smell". The Guardian.
- ^ "Preparation of furfuryl mercaptane". Organic Syntheses. 35: 66. 1955. doi:10.15227/orgsyn.035.0066.
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk
