A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products.[1] An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction.
An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reversible inhibitors produce different types of inhibition depending on whether they bind to the enzyme, the enzyme-substrate complex, or both.
Enzyme inhibitors play an important role in all cells, since they are generally specific to one enzyme each and serve to control that enzyme's activity. For example, enzymes in a metabolic pathway may be inhibited by molecules produced later in the pathway, thus curtailing the production of molecules that are no longer needed. This type of negative feedback is an important way to maintain balance in a cell.[2] Enzyme inhibitors also control essential enzymes such as proteases or nucleases that, if left unchecked, may damage a cell. Many poisons produced by animals or plants are enzyme inhibitors that block the activity of crucial enzymes in prey or predators.
Many drug molecules are enzyme inhibitors that inhibit an aberrant human enzyme or an enzyme critical for the survival of a pathogen such as a virus, bacterium or parasite. Examples include methotrexate (used in chemotherapy and in treating rheumatic arthritis) and the protease inhibitors used to treat HIV/AIDS. Since anti-pathogen inhibitors generally target only one enzyme, such drugs are highly specific and generally produce few side effects in humans, provided that no analogous enzyme is found in humans. (This is often the case, since such pathogens and humans are genetically distant.) Medicinal enzyme inhibitors often have low dissociation constants, meaning that only a minute amount of the inhibitor is required to inhibit the enzyme. A low concentration of the enzyme inhibitor reduces the risk for liver and kidney damage and other adverse drug reactions in humans. Hence the discovery and refinement of enzyme inhibitors is an active area of research in biochemistry and pharmacology.
Structural classes
Enzyme inhibitors are a chemically diverse set of substances that range in size from organic small molecules to macromolecular proteins.
Small molecule inhibitors include essential primary metabolites that inhibit upstream enzymes that produce those metabolites. This provides a negative feedback loop that prevents over production of metabolites and thus maintains cellular homeostasis (steady internal conditions).[3][2] Small molecule enzyme inhibitors also include secondary metabolites, which are not essential to the organism that produces them, but provide the organism with an evolutionary advantage, in that they can be used to repel predators or competing organisms or immobilize prey.[4] In addition, many drugs are small molecule enzyme inhibitors that target either disease-modifying enzymes in the patient[1]: 5 or enzymes in pathogens which are required for the growth and reproduction of the pathogen.[5]
In addition to small molecules, some proteins act as enzyme inhibitors. The most prominent example are serpins (serine protease inhibitors) which are produced by animals to protect against inappropriate enzyme activation and by plants to prevent predation.[6] Another class of inhibitor proteins is the ribonuclease inhibitors, which bind to ribonucleases in one of the tightest known protein–protein interactions.[7] A special case of protein enzyme inhibitors are zymogens that contain an autoinhibitory N-terminal peptide that binds to the active site of enzyme that intramolecularly blocks its activity as a protective mechanism against uncontrolled catalysis. The N‑terminal peptide is cleaved (split) from the zymogen enzyme precursor by another enzyme to release an active enzyme.[8]
The binding site of inhibitors on enzymes is most commonly the same site that binds the substrate of the enzyme. These active site inhibitors are known as orthosteric ("regular" orientation) inhibitors.[9] The mechanism of orthosteric inhibition is simply to prevent substrate binding to the enzyme through direct competition which in turn prevents the enzyme from catalysing the conversion of substrates into products. Alternatively, the inhibitor can bind to a site remote from the enzyme active site. These are known as allosteric ("alternative" orientation) inhibitors.[9] The mechanisms of allosteric inhibition are varied and include changing the conformation (shape) of the enzyme such that it can no longer bind substrate (kinetically indistinguishable from competitive orthosteric inhibition)[10] or alternatively stabilise binding of substrate to the enzyme but lock the enzyme in a conformation which is no longer catalytically active.[11]
Reversible inhibitors
Reversible inhibitors attach to enzymes with non-covalent interactions such as hydrogen bonds, hydrophobic interactions and ionic bonds.[12] Multiple weak bonds between the inhibitor and the enzyme active site combine to produce strong and specific binding.
In contrast to irreversible inhibitors, reversible inhibitors generally do not undergo chemical reactions when bound to the enzyme and can be easily removed by dilution or dialysis. A special case is covalent reversible inhibitors that form a chemical bond with the enzyme, but the bond can be cleaved so the inhibition is fully reversible.[13]
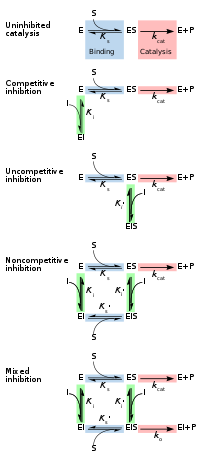
Reversible inhibitors are generally categorized into four types, as introduced by Cleland in 1963.[14] They are classified according to the effect of the inhibitor on the Vmax (maximum reaction rate catalysed by the enzyme) and Km (the concentration of substrate resulting in half maximal enzyme activity) as the concentration of the enzyme's substrate is varied.[15][16]
Competitive
In competitive inhibition the substrate and inhibitor cannot bind to the enzyme at the same time.[17]: 134 This usually results from the inhibitor having an affinity for the active site of an enzyme where the substrate also binds; the substrate and inhibitor compete for access to the enzyme's active site. This type of inhibition can be overcome by sufficiently high concentrations of substrate (Vmax remains constant), i.e., by out-competing the inhibitor.[17]: 134–135 However, the apparent Km will increase as it takes a higher concentration of the substrate to reach the Km point, or half the Vmax. Competitive inhibitors are often similar in structure to the real substrate (see for example the "methotrexate versus folate" figure in the "Drugs" section).[17]: 134
Uncompetitive
In uncompetitive inhibition the inhibitor binds only to the enzyme-substrate complex.[17]: 139 This type of inhibition causes Vmax to decrease (maximum velocity decreases as a result of removing activated complex) and Km to decrease (due to better binding efficiency as a result of Le Chatelier's principle and the effective elimination of the ES complex thus decreasing the Km which indicates a higher binding affinity).[18] Uncompetitive inhibition is rare.[17]: 139 [19]
Non-competitive
In non-competitive inhibition the binding of the inhibitor to the enzyme reduces its activity but does not affect the binding of substrate.[16] This type of inhibitor binds with equal affinity to the free enzyme as to the enzyme-substrate complex. It can be thought of as having the ability of competitive and uncompetitive inhibitors, but with no preference to either type. As a result, the extent of inhibition depends only on the concentration of the inhibitor. Vmax will decrease due to the inability for the reaction to proceed as efficiently, but Km will remain the same as the actual binding of the substrate, by definition, will still function properly.[20]
Mixed
In mixed inhibition the inhibitor may bind to the enzyme whether or not the substrate has already bound. Hence mixed inhibition is a combination of competitive and noncompetitive inhibition.[16] Furthermore, the affinity of the inhibitor for the free enzyme and the enzyme-substrate complex may differ.[17]: 136–139 By increasing concentrations of substrate , this type of inhibition can be reduced (due to the competitive contribution), but not entirely overcome (due to the noncompetitive component).[21]: 381–382 Although it is possible for mixed-type inhibitors to bind in the active site, this type of inhibition generally results from an allosteric effect where the inhibitor binds to a different site on an enzyme. Inhibitor binding to this allosteric site changes the conformation (that is, the tertiary structure or three-dimensional shape) of the enzyme so that the affinity of the substrate for the active site is reduced.[22]
These four types of inhibition can also be distinguished by the effect of increasing the substrate concentration on the degree of inhibition caused by a given amount of inhibitor. For competitive inhibition the degree of inhibition is reduced by increasing , for noncompetitive inhibition the degree of inhibition is unchanged, and for uncompetitive (also called anticompetitive) inhibition the degree of inhibition increases with .[23]
Quantitative description
Reversible inhibition can be described quantitatively in terms of the inhibitor's binding to the enzyme and to the enzyme-substrate complex, and its effects on the kinetic constants of the enzyme.[24]: 6 In the classic Michaelis-Menten scheme (shown in the "inhibition mechanism schematic" diagram), an enzyme (E) binds to its substrate (S) to form the enzyme–substrate complex ES. Upon catalysis, this complex breaks down to release product P and free enzyme.[24]: 55 The inhibitor (I) can bind to either E or ES with the dissociation constants Ki or Ki', respectively.[24]: 87
- Competitive inhibitors can bind to E, but not to ES. Competitive inhibition increases Km (i.e., the inhibitor interferes with substrate binding), but does not affect Vmax (the inhibitor does not hamper catalysis in ES because it cannot bind to ES).[24]: 102
- Uncompetitive inhibitors bind to ES. Uncompetitive inhibition decreases both Km and Vmax. The inhibitor affects substrate binding by increasing the enzyme's affinity for the substrate (decreasing Km) as well as hampering catalysis (decreases Vmax).[24]: 106
- Non-competitive inhibitors have identical affinities for E and ES (Ki = Ki'). Non-competitive inhibition does not change Km (i.e., it does not affect substrate binding) but decreases Vmax (i.e., inhibitor binding hampers catalysis).[24]: 97
- Mixed-type inhibitors bind to both E and ES, but their affinities for these two forms of the enzyme are different (Ki ≠ Ki'). Thus, mixed-type inhibitors affect substrate binding (increase or decrease Km) and hamper catalysis in the ES complex (decrease Vmax).[25]: 63–64
When an enzyme has multiple substrates, inhibitors can show different types of inhibition depending on which substrate is considered. This results from the active site containing two different binding sites within the active site, one for each substrate. For example, an inhibitor might compete with substrate A for the first binding site, but be a non-competitive inhibitor with respect to substrate B in the second binding site.[26]
Traditionally reversible enzyme inhibitors have been classified as competitive, uncompetitive, or non-competitive, according to their effects on Km and Vmax.[14] These three types of inhibition result respectively from the inhibitor binding only to the enzyme E in the absence of substrate S, to the enzyme–substrate complex ES, or to both. The division of these classes arises from a problem in their derivation and results in the need to use two different binding constants for one binding event.[27] It is further assumed that binding of the inhibitor to the enzyme results in 100% inhibition and fails to consider the possibility of partial inhibition.[27] The common form of the inhibitory term also obscures the relationship between the inhibitor binding to the enzyme and its relationship to any other binding term be it the Michaelis–Menten equation or a dose response curve associated with ligand receptor binding. To demonstrate the relationship the following rearrangement can be made:[28]
This rearrangement demonstrates that similar to the Michaelis–Menten equation, the maximal rate of reaction depends on the proportion of the enzyme population interacting with its substrate.
fraction of the enzyme population bound by substrate
fraction of the enzyme population bound by inhibitor
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk