
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric/humanized hybrid (mouse/human) |
| Target | EGFR |
| Clinical data | |
| Other names | ABT-414 |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6624H10228N1728O2052S42 |
| Molar mass | 148251.25 g·mol−1 |
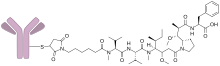
Depatuxizumab mafodotin (INN; development code ABT-414) is an antibody-drug conjugate designed for the treatment of cancer.[1][2] It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.[3][4]
This drug was developed by AbbVie. In May 2019 AbbVie stopped enrolment in all studies of Depatux-M after late-stage failure in newly diagnosed glioblastoma.[5]
In 2014, Orphan Drug Status was granted by the FDA for glioblastoma multiforme.[6] It is in phase II/III clinical trials for glioblastoma, in phase II clinical trials for non-small cell lung cancer, and in phase I clinical trials for the treatment of other solid tumors.[7] Phase I results were presented at ASCO in 2016.[7]
References
- ^ Statement On A Nonproprietary Name Adopted By The USAN Council - Depatuxizumab Mafodotin, American Medical Association.
- ^ World Health Organization (2016). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 115" (PDF). WHO Drug Information. 30 (2).
- ^ "Antibody-drug conjugates in the spotlight - The Antibody Society". antibodysociety.org. 14 October 2016.
- ^ Nagayama A, Ellisen LW, Chabner B, Bardia A (December 2017). "Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments". Targeted Oncology. 12 (6): 719–739. doi:10.1007/s11523-017-0535-0. PMID 29116596. S2CID 1012740.
- ^ "AbbVie Provides Update on Depatuxizumab Mafodotin (Depatux-M), an Investigational Medicine for Newly Diagnosed Glioblastoma, an Aggressive Form of Brain Cancer - PM AbbVie, May 17, 2019". abbvie.com. Retrieved 20 May 2019.
- ^ "Depatuxizumab mafodotin". en.pharmacodia.com.
- ^ a b "Depatuxizumab mafodotin - AbbVie - AdisInsight". adisinsight.springer.com.
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk
