
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
The bisphenols (/ˈbɪsfɪnɒl/) are a group of chemical compounds related to diphenylmethane. Most are based on two hydroxyphenyl functional groups linked by a methylene bridge. Exceptions include bisphenol S, P, and M. "Bisphenol" is a common name; the letter following denotes the variant, which depends on the additional substituents. Bisphenol A is the most popular representative of the group, often simply called "bisphenol".[1]
List
| Structural formula | Name | CAS | Reactants | |
|---|---|---|---|---|
| Bisphenol A | 80-05-7 | Phenol | Acetone | |
 |
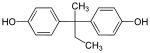
Bisphenol AP | 1571-75-1 | Phenol | Acetophenone |
 |
Bisphenol AF | 1478-61-1 | Phenol | Hexafluoroacetone |
 |
Bisphenol B | 77-40-7 | Phenol | Butanone |
 |
Bisphenol BP | 1844-01-5 | Phenol | Benzophenone |
 |
Bisphenol C | 79-97-0 | o-cresol | Acetone |
 |
Bisphenol C 2 | 14868-03-2 | Phenol | Chloral |
| Bisphenol E | 2081-08-5 | Phenol | Ethanal | |
| Bisphenol F | 620-92-8 | Phenol | Formaldehyde | |
 |
Bisphenol G | 127-54-8 | 2-Isopropylphenol | Acetone |
| Bisphenol M | 13595-25-0 | |||
 |
Bisphenol S | 80-09-1 | Phenol | Sulfur trioxide |
 |
Bisphenol P | 2167-51-3 | ||
 |
Bisphenol PH | 24038-68-4 | 2-Phenylphenol | Acetone |
 |
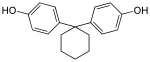
Bisphenol TMC | 129188-99-4 | Phenol | 3,3,5-Trimethylcyclohexanone |
 |
Bisphenol Z | 843-55-0 | Phenol | Cyclohexanone |
 |
Dinitrobisphenol A | 5329-21-5 | Bisphenol A | Nitric acid |
 |
Tetrabromobisphenol A | 79-94-7 | Bisphenol A | Bromine |
Health effects
Bisphenols A (BPA), F (BPF) and S (BPS) have been shown to be endocrine disruptors.[2] Due to its high production volumes, BPA has been characterised as a "pseudo-persistent" chemical,[3] leading to its spreading and potential accumulation in a variety of environmental matrices, even though it has a fairly short half-life.[4]
References
- ^ Helmut Fiege; Heinz-Werner Voges; Toshikazu Hamamoto; Sumio Umemura; Tadao Iwata; Hisaya Miki; Yasuhiro Fujita; Hans-Josef Buysch; Dorothea Garbe; Wilfried Paulus (2002). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3-527-30673-2..
- ^ Bilbrey, Jenna (11 August 2014). "BPA-Free Plastic Containers May Be Just as Hazardous". Scientific American. Retrieved 8 August 2015.
- ^ Pivnenko, K.; Pedersen, G. A.; Eriksson, E.; Astrup, T. F. (2015). "Bisphenol A and its structural analogues in household waste paper" (PDF). Waste Management. 44: 39–47. doi:10.1016/j.wasman.2015.07.017. PMID 26194879. S2CID 217938141.
- ^ See Bisphenol A#Environmental effects for extensive discussion
- For additional examples and alternate names, see: Alger, Mark (2017). Polymer Science Dictionary. Springer. p. 77. ISBN 978-94-024-0893-5.
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk
